Dynein interacts with the neural cell adhesion molecule (NCAM180) to tether dynamic microtubules and maintain synaptic density in cortical neurons
- PMID: 23960070
- PMCID: PMC3784697
- DOI: 10.1074/jbc.M113.465088
Dynein interacts with the neural cell adhesion molecule (NCAM180) to tether dynamic microtubules and maintain synaptic density in cortical neurons
Abstract
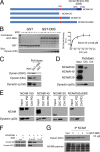
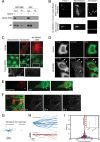
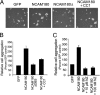
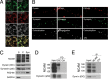
Cytoplasmic dynein is well characterized as an organelle motor, but dynein also acts to tether and stabilize dynamic microtubule plus-ends in vitro. Here we identify a novel and direct interaction between dynein and the 180-kDa isoform of the neural cell adhesion molecule (NCAM). Optical trapping experiments indicate that dynein bound to beads via the NCAM180 interaction domain can tether projecting microtubule plus-ends. Live cell assays indicate that the NCAM180-dependent recruitment of dynein to the cortex leads to the selective stabilization of microtubules projecting to NCAM180 patches at the cell periphery. The dynein-NCAM180 interaction also enhances cell-cell adhesion in heterologous cell assays. Dynein and NCAM180 co-precipitate from mouse brain extract and from synaptosomal fractions, consistent with an endogenous interaction in neurons. Thus, we examined microtubule dynamics and synaptic density in primary cortical neurons. We find that depletion of NCAM, inhibition of the dynein-NCAM180 interaction, or dampening of microtubule dynamics with low dose nocodazole all result in significantly decreased in synaptic density. Based on these observations, we propose a working model for the role of dynein at the synapse, in which the anchoring of the motor to the cortex via binding to an adhesion molecule mediates the tethering of dynamic microtubule plus-ends to potentiate synaptic stabilization.
Keywords: Cytoskeleton; Dynamic Instability; Dynein; Microtubules; NCAM; Synapses; Synaptosomes.
Figures
Similar articles
-
Dynein tethers and stabilizes dynamic microtubule plus ends.Curr Biol. 2012 Apr 10;22(7):632-7. doi: 10.1016/j.cub.2012.02.023. Epub 2012 Mar 22. Curr Biol. 2012. PMID: 22445300 Free PMC article.
-
DYNLT (Tctex-1) forms a tripartite complex with dynein intermediate chain and RagA, hence linking this small GTPase to the dynein motor.FEBS J. 2015 Oct;282(20):3945-58. doi: 10.1111/febs.13388. Epub 2015 Aug 20. FEBS J. 2015. PMID: 26227614
-
UCHL1 regulates ubiquitination and recycling of the neural cell adhesion molecule NCAM.FEBS J. 2012 Dec;279(23):4398-409. doi: 10.1111/febs.12029. Epub 2012 Nov 8. FEBS J. 2012. PMID: 23061666
-
Molecular organization and force-generating mechanism of dynein.FEBS J. 2011 Sep;278(17):2964-79. doi: 10.1111/j.1742-4658.2011.08253.x. Epub 2011 Aug 8. FEBS J. 2011. PMID: 21777385 Review.
-
The Hidden Side of NCAM Family: NCAM2, a Key Cytoskeleton Organization Molecule Regulating Multiple Neural Functions.Int J Mol Sci. 2021 Sep 16;22(18):10021. doi: 10.3390/ijms221810021. Int J Mol Sci. 2021. PMID: 34576185 Free PMC article. Review.
Cited by
-
Reciprocal Interactions between Cell Adhesion Molecules of the Immunoglobulin Superfamily and the Cytoskeleton in Neurons.Front Cell Dev Biol. 2016 Feb 16;4:9. doi: 10.3389/fcell.2016.00009. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 26909348 Free PMC article. Review.
-
Local inhibition of microtubule dynamics by dynein is required for neuronal cargo distribution.Nat Commun. 2017 Apr 13;8:15063. doi: 10.1038/ncomms15063. Nat Commun. 2017. PMID: 28406181 Free PMC article.
-
Dynein-driven regulation of postsynaptic membrane architecture and synaptic function.J Cell Sci. 2025 Mar 1;138(5):JCS263844. doi: 10.1242/jcs.263844. Epub 2025 Mar 12. J Cell Sci. 2025. PMID: 39865922 Free PMC article.
-
Dynein disruption perturbs post-synaptic components and contributes to impaired MuSK clustering at the NMJ: implication in ALS.Sci Rep. 2016 Jun 10;6:27804. doi: 10.1038/srep27804. Sci Rep. 2016. PMID: 27283349 Free PMC article.
-
The Dynamic Localization of Cytoplasmic Dynein in Neurons Is Driven by Kinesin-1.Neuron. 2016 Jun 1;90(5):1000-15. doi: 10.1016/j.neuron.2016.04.046. Epub 2016 May 19. Neuron. 2016. PMID: 27210554 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

