JNK3 enzyme binding to arrestin-3 differentially affects the recruitment of upstream mitogen-activated protein (MAP) kinase kinases
- PMID: 23960075
- PMCID: PMC3789954
- DOI: 10.1074/jbc.M113.508085
JNK3 enzyme binding to arrestin-3 differentially affects the recruitment of upstream mitogen-activated protein (MAP) kinase kinases
Abstract
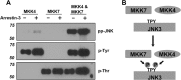
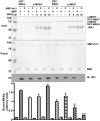
Arrestin-3 was previously shown to bind JNK3α2, MKK4, and ASK1. However, full JNK3α2 activation requires phosphorylation by both MKK4 and MKK7. Using purified proteins we show that arrestin-3 directly interacts with MKK7 and promotes JNK3α2 phosphorylation by both MKK4 and MKK7 in vitro as well as in intact cells. The binding of JNK3α2 promotes an arrestin-3 interaction with MKK4 while reducing its binding to MKK7. Interestingly, the arrestin-3 concentration optimal for scaffolding the MKK7-JNK3α2 module is ∼10-fold higher than for the MKK4-JNK3α2 module. The data provide a mechanistic basis for arrestin-3-dependent activation of JNK3α2. The opposite effects of JNK3α2 on arrestin-3 interactions with MKK4 and MKK7 is the first demonstration that the kinase components in mammalian MAPK cascades regulate each other's interactions with a scaffold protein. The results show how signaling outcomes can be affected by the relative expression of scaffolding proteins and components of signaling cascades that they assemble.
Keywords: Arrestin; Cell Signaling; Jun N-terminal Kinase (JNK); MAP Kinases (MAPKs); Protein Kinases; Protein Phosphorylation.
Figures





References
-
- DeWire S. M., Ahn S., Lefkowitz R. J., Shenoy S. K. (2007) β-Arrestins and cell signaling. Annu. Rev. Physiol. 69, 483–510 - PubMed
-
- Pearson G., Robinson F., Beers Gibson T., Xu B. E., Karandikar M., Berman K., Cobb M. H. (2001) Mitogen-activated protein (MAP) kinase pathways. Regulation and physiological functions. Endocr. Rev. 22, 153–183 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

