Structural and functional properties of the membranotropic HIV-1 glycoprotein gp41 loop region are modulated by its intrinsic hydrophobic core
- PMID: 23960077
- PMCID: PMC3790013
- DOI: 10.1074/jbc.M113.496646
Structural and functional properties of the membranotropic HIV-1 glycoprotein gp41 loop region are modulated by its intrinsic hydrophobic core
Abstract
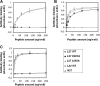
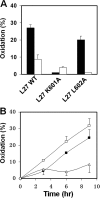
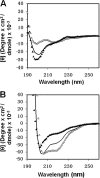
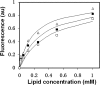
The gp41 disulfide loop region switches from a soluble state to a membrane-bound state during the human immunodeficiency virus type 1 (HIV-1) envelope-mediated membrane fusion process. The loop possesses a hydrophobic core at the center of the region with an unusual basic residue (Lys-601). Furthermore, two loop core mutations, K601A and L602A, are found to inhibit HIV-1 infectivity while keeping wild type-like levels of the envelope, implying that they exert an inhibitory effect on gp41 during the membrane fusion event. Here, we investigated the mode of action of these mutations on the loop region. We show that the K601A mutation, but not the L602A mutation, abolished the binding of a loop-specific monoclonal antibody to a loop domain peptide. Additionally, the K601A, but not the L602A, impaired disulfide bond formation in the peptides. This was correlated with changes in the circular dichroism spectrum imposed by the K601A mutation. In the membrane, however, the L602A, but not the K601A, reduced the lipid mixing ability of the loop peptides, which was correlated with decreased α-helical content of the L602A mutant. The results suggest that the Lys-601 residue provides a moderate hydrophobicity level within the gp41 loop core that contributes to the proper structure and function of the loop inside and outside the membrane. Because basic residues are found between the loop Cys residues of several lentiviral fusion proteins, the findings may contribute to understanding the fusion mechanism of other viruses as well.
Keywords: Biophysics; HIV-1; Membrane Fusion; Membrane Proteins; Peptide Conformation; Peptide-Membrane Interaction; Viral Fusion Protein.
Figures

Similar articles
-
Functional analysis of the disulfide-bonded loop/chain reversal region of human immunodeficiency virus type 1 gp41 reveals a critical role in gp120-gp41 association.J Virol. 2001 Jul;75(14):6635-44. doi: 10.1128/JVI.75.14.6635-6644.2001. J Virol. 2001. PMID: 11413331 Free PMC article.
-
Role of the ectodomain of the gp41 transmembrane envelope protein of human immunodeficiency virus type 1 in late steps of the membrane fusion process.J Virol. 2004 Jan;78(2):811-20. doi: 10.1128/jvi.78.2.811-820.2004. J Virol. 2004. PMID: 14694113 Free PMC article.
-
Genetic evidence that interhelical packing interactions in the gp41 core are critical for transition of the human immunodeficiency virus type 1 envelope glycoprotein to the fusion-active state.J Virol. 2002 Jul;76(14):7356-62. doi: 10.1128/jvi.76.14.7356-7362.2002. J Virol. 2002. PMID: 12072535 Free PMC article.
-
Fusion peptides derived from the HIV type 1 glycoprotein 41 associate within phospholipid membranes and inhibit cell-cell Fusion. Structure-function study.J Biol Chem. 1997 May 23;272(21):13496-505. doi: 10.1074/jbc.272.21.13496. J Biol Chem. 1997. PMID: 9153194
-
Peptide and non-peptide HIV fusion inhibitors.Curr Pharm Des. 2002;8(8):563-80. doi: 10.2174/1381612024607180. Curr Pharm Des. 2002. PMID: 11945159 Review.
Cited by
-
Statistical correlation of nonconservative substitutions of HIV gp41 variable amino acid residues with the R5X4 HIV-1 phenotype.Virol J. 2016 Feb 16;13:28. doi: 10.1186/s12985-016-0486-6. Virol J. 2016. PMID: 26879054 Free PMC article.
-
Disulfide Sensitivity in the Env Protein Underlies Lytic Inactivation of HIV-1 by Peptide Triazole Thiols.ACS Chem Biol. 2015 Dec 18;10(12):2861-73. doi: 10.1021/acschembio.5b00381. Epub 2015 Oct 22. ACS Chem Biol. 2015. PMID: 26458166 Free PMC article.
-
Peptide Triazole Thiol Irreversibly Inactivates Metastable HIV-1 Env by Accessing Conformational Triggers Intrinsic to Virus-Cell Entry.Microorganisms. 2021 Jun 12;9(6):1286. doi: 10.3390/microorganisms9061286. Microorganisms. 2021. PMID: 34204725 Free PMC article.
References
-
- White J. M. (1992) Membrane fusion. Science 258, 917–924 - PubMed
-
- Poranen M. M., Daugelavicius R., Bamford D. H. (2002) Common principles in viral entry. Annu. Rev. Microbiol. 56, 521–538 - PubMed
-
- Eckert D. M., Kim P. S. (2001) Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70, 777–810 - PubMed
-
- Gallo S. A., Finnegan C. M., Viard M., Raviv Y., Dimitrov A., Rawat S. S., Puri A., Durell S., Blumenthal R. (2003) The HIV Env-mediated fusion reaction. Biochim Biophys. Acta 1614, 36–50 - PubMed
-
- Colman P. M., Lawrence M. C. (2003) The structural biology of type I viral membrane fusion. Nat. Rev. Mol. Cell Biol. 4, 309–319 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

