Mechanism for recognition of an unusual mycobacterial glycolipid by the macrophage receptor mincle
- PMID: 23960080
- PMCID: PMC3789947
- DOI: 10.1074/jbc.M113.497149
Mechanism for recognition of an unusual mycobacterial glycolipid by the macrophage receptor mincle
Abstract
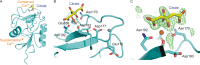
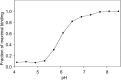
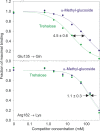
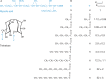
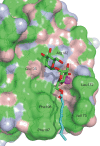
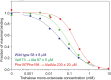
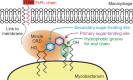
Binding of the macrophage lectin mincle to trehalose dimycolate, a key glycolipid virulence factor on the surface of Mycobacterium tuberculosis and Mycobacterium bovis, initiates responses that can lead both to toxicity and to protection of these pathogens from destruction. Crystallographic structural analysis, site-directed mutagenesis, and binding studies with glycolipid mimics have been used to define an extended binding site in the C-type carbohydrate recognition domain (CRD) of bovine mincle that encompasses both the headgroup and a portion of the attached acyl chains. One glucose residue of the trehalose Glcα1-1Glcα headgroup is liganded to a Ca(2+) in a manner common to many C-type CRDs, whereas the second glucose residue is accommodated in a novel secondary binding site. The additional contacts in the secondary site lead to a 36-fold higher affinity for trehalose compared with glucose. An adjacent hydrophobic groove, not seen in other C-type CRDs, provides a docking site for one of the acyl chains attached to the trehalose, which can be targeted with small molecule analogs of trehalose dimycolate that bind with 52-fold higher affinity than trehalose. The data demonstrate how mincle bridges between the surfaces of the macrophage and the mycobacterium and suggest the possibility of disrupting this interaction. In addition, the results may provide a basis for design of adjuvants that mimic the ability of mycobacteria to stimulate a response to immunization that can be employed in vaccine development.
Keywords: CLEC4E; Carbohydrate-binding Protein; Cord Factor; Crystal Structure; Glycobiology; Glycolipids; Mycobacterium tuberculosis.
Figures



Similar articles
-
Defining the conformation of human mincle that interacts with mycobacterial trehalose dimycolate.Glycobiology. 2014 Dec;24(12):1291-300. doi: 10.1093/glycob/cwu072. Epub 2014 Jul 15. Glycobiology. 2014. PMID: 25028392 Free PMC article.
-
Binding Sites for Acylated Trehalose Analogs of Glycolipid Ligands on an Extended Carbohydrate Recognition Domain of the Macrophage Receptor Mincle.J Biol Chem. 2016 Sep 30;291(40):21222-21233. doi: 10.1074/jbc.M116.749515. Epub 2016 Aug 19. J Biol Chem. 2016. PMID: 27542410 Free PMC article.
-
Structural analysis for glycolipid recognition by the C-type lectins Mincle and MCL.Proc Natl Acad Sci U S A. 2013 Oct 22;110(43):17438-43. doi: 10.1073/pnas.1312649110. Epub 2013 Oct 7. Proc Natl Acad Sci U S A. 2013. PMID: 24101491 Free PMC article.
-
Immune Recognition of Pathogen-Derived Glycolipids Through Mincle.Adv Exp Med Biol. 2020;1204:31-56. doi: 10.1007/978-981-15-1580-4_2. Adv Exp Med Biol. 2020. PMID: 32152942 Review.
-
Mincle: 20 years of a versatile sensor of insults.Int Immunol. 2018 May 24;30(6):233-239. doi: 10.1093/intimm/dxy028. Int Immunol. 2018. PMID: 29726997 Review.
Cited by
-
The C-type lectin receptor Mincle binds to Streptococcus pneumoniae but plays a limited role in the anti-pneumococcal innate immune response.PLoS One. 2015 Feb 6;10(2):e0117022. doi: 10.1371/journal.pone.0117022. eCollection 2015. PLoS One. 2015. PMID: 25658823 Free PMC article.
-
Commentary: Modification of Host Responses by Mycobacteria.Front Immunol. 2017 Apr 28;8:466. doi: 10.3389/fimmu.2017.00466. eCollection 2017. Front Immunol. 2017. PMID: 28503174 Free PMC article. No abstract available.
-
Probing Isosteric Replacement for Immunoadjuvant Design: Bis-Aryl Triazole Trehalolipids are Mincle Agonists.ACS Med Chem Lett. 2024 May 21;15(6):899-905. doi: 10.1021/acsmedchemlett.4c00100. eCollection 2024 Jun 13. ACS Med Chem Lett. 2024. PMID: 38894898 Free PMC article.
-
C-Type Lectin Receptor (CLR)-Fc Fusion Proteins As Tools to Screen for Novel CLR/Bacteria Interactions: An Exemplary Study on Preselected Campylobacter jejuni Isolates.Front Immunol. 2018 Feb 13;9:213. doi: 10.3389/fimmu.2018.00213. eCollection 2018. Front Immunol. 2018. PMID: 29487596 Free PMC article.
-
Host Responses to Malassezia spp. in the Mammalian Skin.Front Immunol. 2017 Nov 22;8:1614. doi: 10.3389/fimmu.2017.01614. eCollection 2017. Front Immunol. 2017. PMID: 29213272 Free PMC article. Review.
References
-
- Maartens G., Wilkinson R. J. (2007) Tuberculosis. Lancet 370, 2030–2043 - PubMed
-
- Gilbert M., Mitchell A., Bourn D., Mawdsley J., Clifton-Hadley R., Wint W. (2005) Cattle movements and bovine tuberculosis in Great Britain. Nature 435, 491–496 - PubMed
-
- Matsumoto M., Tanaka T., Kaisho T., Sanjo H., Copeland N. G., Gilbert D. J., Jenkins N. A., Akira S. (1999) A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J. Immunol. 163, 5039–5048 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

