Identification of ectonucleotide pyrophosphatase/phosphodiesterase 3 (ENPP3) as a regulator of N-acetylglucosaminyltransferase GnT-IX (GnT-Vb)
- PMID: 23960081
- PMCID: PMC3784706
- DOI: 10.1074/jbc.M113.474304
Identification of ectonucleotide pyrophosphatase/phosphodiesterase 3 (ENPP3) as a regulator of N-acetylglucosaminyltransferase GnT-IX (GnT-Vb)
Abstract
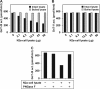
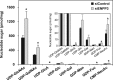
Our previous studies on a β1,6-N-acetylglucosaminyltransferase, GnT-IX (GnT-Vb), a homolog of GnT-V, indicated that the enzyme has a broad GlcNAc transfer activity toward N-linked and O-mannosyl glycan core structures and that its brain-specific gene expression is regulated by epigenetic histone modifications. In this study, we demonstrate the existence of an endogenous inhibitory factor for GnT-IX that functions as a key regulator for GnT-IX enzymatic activity in Neuro2a (N2a) cells. We purified this factor from N2a cells and found that it is identical to ectonucleotide pyrophosphatase/phosphodiesterase 3 (ENPP3), as evidenced by mass spectrometry and by the knockdown and overexpression of ENPP3 in cultured cells. Kinetic analyses revealed that the mechanism responsible for the inhibition of GnT-IX caused by ENPP3 is the ENPP3-mediated hydrolysis of the nucleotide sugar donor substrate, UDP-GlcNAc, with the resulting generation of UMP, a potent and competitive inhibitor of GnT-IX. Indeed, ENPP3 knockdown cells had significantly increased levels of intracellular nucleotide sugars and displayed changes in the total cellular glycosylation profile. In addition to chaperones or other known regulators of glycosyltransferases, the ENPP3-mediated hydrolysis of nucleotide sugars would have widespread and significant impacts on glycosyltransferase activities and would be responsible for altering the total cellular glycosylation profile and modulating cellular functions.
Keywords: Glycobiology; Glycosylation; Glycosyltransferases; Nucleoside Nucleotide Metabolism; Phosphodiesterases.
Figures






Similar articles
-
Developmental expression of the neuron-specific N-acetylglucosaminyltransferase Vb (GnT-Vb/IX) and identification of its in vivo glycan products in comparison with those of its paralog, GnT-V.J Biol Chem. 2012 Aug 17;287(34):28526-36. doi: 10.1074/jbc.M112.367565. Epub 2012 Jun 19. J Biol Chem. 2012. PMID: 22715095 Free PMC article.
-
Epigenetic regulation of a brain-specific glycosyltransferase N-acetylglucosaminyltransferase-IX (GnT-IX) by specific chromatin modifiers.J Biol Chem. 2014 Apr 18;289(16):11253-11261. doi: 10.1074/jbc.M114.554311. Epub 2014 Mar 10. J Biol Chem. 2014. PMID: 24619417 Free PMC article.
-
Integrin-dependent neuroblastoma cell adhesion and migration on laminin is regulated by expression levels of two enzymes in the O-mannosyl-linked glycosylation pathway, PomGnT1 and GnT-Vb.Exp Cell Res. 2006 Sep 10;312(15):2837-50. doi: 10.1016/j.yexcr.2006.05.022. Epub 2006 Jun 21. Exp Cell Res. 2006. PMID: 16857188
-
Glycans and cancer: role of N-glycans in cancer biomarker, progression and metastasis, and therapeutics.Adv Cancer Res. 2015;126:11-51. doi: 10.1016/bs.acr.2014.11.001. Epub 2015 Feb 7. Adv Cancer Res. 2015. PMID: 25727145 Review.
-
Regulation of intracellular activity of N-glycan branching enzymes in mammals.J Biol Chem. 2024 Jul;300(7):107471. doi: 10.1016/j.jbc.2024.107471. Epub 2024 Jun 13. J Biol Chem. 2024. PMID: 38879010 Free PMC article. Review.
Cited by
-
β-Lactoglobulin affects the oxidative status and viability of equine endometrial progenitor cells via lncRNA-mRNA-miRNA regulatory associations.J Cell Mol Med. 2023 Apr;27(7):927-938. doi: 10.1111/jcmm.17694. Epub 2023 Mar 1. J Cell Mol Med. 2023. PMID: 36860157 Free PMC article.
-
Antibody-Drug Conjugates for the Treatment of Renal Cancer: A Scoping Review on Current Evidence and Clinical Perspectives.J Pers Med. 2023 Aug 30;13(9):1339. doi: 10.3390/jpm13091339. J Pers Med. 2023. PMID: 37763107 Free PMC article. Review.
-
Crystal structure and substrate binding mode of ectonucleotide phosphodiesterase/pyrophosphatase-3 (NPP3).Sci Rep. 2018 Jul 18;8(1):10874. doi: 10.1038/s41598-018-28814-y. Sci Rep. 2018. PMID: 30022031 Free PMC article.
-
A Proteome Approach Reveals Differences between Fertile Women and Patients with Repeated Implantation Failure on Endometrial Level⁻Does hCG Render the Endometrium of RIF Patients?Int J Mol Sci. 2019 Jan 19;20(2):425. doi: 10.3390/ijms20020425. Int J Mol Sci. 2019. PMID: 30669470 Free PMC article.
-
Regulation of allergic inflammation by the ectoenzyme E-NPP3 (CD203c) on basophils and mast cells.Semin Immunopathol. 2016 Sep;38(5):571-9. doi: 10.1007/s00281-016-0564-2. Epub 2016 Apr 30. Semin Immunopathol. 2016. PMID: 27130555 Review.
References
-
- Apweiler R., Hermjakob H., Sharon N. (1999) On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim. Biophys. Acta 1473, 4–8 - PubMed
-
- Ohtsubo K., Marth J. D. (2006) Glycosylation in cellular mechanisms of health and disease. Cell 126, 855–867 - PubMed
-
- Taniguchi N., Miyoshi E., Gu J., Jianguo G., Honke K., Matsumoto A. (2006) Decoding sugar functions by identifying target glycoproteins. Curr. Opin. Struct. Biol. 16, 561–566 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

