IL-25 simultaneously elicits distinct populations of innate lymphoid cells and multipotent progenitor type 2 (MPPtype2) cells
- PMID: 23960191
- PMCID: PMC3754870
- DOI: 10.1084/jem.20122332
IL-25 simultaneously elicits distinct populations of innate lymphoid cells and multipotent progenitor type 2 (MPPtype2) cells
Abstract
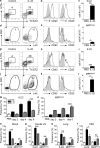
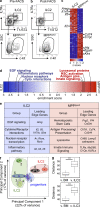
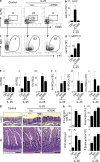
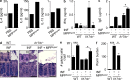
The predominantly epithelial cell-derived cytokines IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) can promote CD4(+) Th2 cell-dependent immunity, inflammation, and tissue repair at barrier surfaces through the induction of multiple innate immune cell populations. IL-25 and IL-33 were previously shown to elicit four innate cell populations, named natural helper cells, nuocytes, innate type 2 helper cells, and multipotent progenitor type 2 (MPP(type2)) cells, now collectively termed group 2 innate lymphoid cells (ILC2). In contrast to other types of ILC2, MPP(type2) cells exhibit multipotent potential and do not express T1/ST2 or IL-7Rα, suggesting that MPP(type2) cells may be a distinct population. Here, we show that IL-33 elicits robust ILC2 responses, whereas IL-25 predominantly promotes MPP(type2) cell responses at multiple tissue sites with limited effects on ILC2 responses. MPP(type2) cells were distinguished from ILC2 by their differential developmental requirements for specific transcription factors, distinct genome-wide transcriptional profile, and functional potential. Furthermore, IL-25-induced MPP(type2) cells promoted Th2 cytokine-associated inflammation after depletion of ILC2. These findings indicate that IL-25 simultaneously elicits phenotypically and functionally distinct innate lymphoid- and nonlymphoid-associated cell populations and implicate IL-25-elicited MPP(type2) cells and extramedullary hematopoiesis in the promotion of Th2 cytokine responses at mucosal surfaces.
Figures




References
-
- Allakhverdi Z., Comeau M.R., Jessup H.K., Yoon B.R., Brewer A., Chartier S., Paquette N., Ziegler S.F., Sarfati M., Delespesse G. 2007. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 204:253–258 10.1084/jem.20062211 - DOI - PMC - PubMed
-
- Allakhverdi Z., Comeau M.R., Smith D.E., Toy D., Endam L.M., Desrosiers M., Liu Y.J., Howie K.J., Denburg J.A., Gauvreau G.M., Delespesse G. 2009. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J. Allergy Clin. Immunol. 123:472–478 10.1016/j.jaci.2008.10.022 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI074878/AI/NIAID NIH HHS/United States
- T32-AI060516/AI/NIAID NIH HHS/United States
- R01 AI095466/AI/NIAID NIH HHS/United States
- AI061570/AI/NIAID NIH HHS/United States
- F32 AI085828/AI/NIAID NIH HHS/United States
- AI087990/AI/NIAID NIH HHS/United States
- R37 AI067545/AI/NIAID NIH HHS/United States
- R21 AI083480/AI/NIAID NIH HHS/United States
- P30-DK050306/DK/NIDDK NIH HHS/United States
- U01 AI095608/AI/NIAID NIH HHS/United States
- R01 AI061570/AI/NIAID NIH HHS/United States
- P30 DK050306/DK/NIDDK NIH HHS/United States
- T32 AI007532/AI/NIAID NIH HHS/United States
- KL2-RR024132/RR/NCRR NIH HHS/United States
- AI102942/AI/NIAID NIH HHS/United States
- AI074878/AI/NIAID NIH HHS/United States
- T32-AI007532/AI/NIAID NIH HHS/United States
- T32 AI060516/AI/NIAID NIH HHS/United States
- 2-P30 CA016520/CA/NCI NIH HHS/United States
- AI083480/AI/NIAID NIH HHS/United States
- R01 AI102942/AI/NIAID NIH HHS/United States
- P30 AR057217/AR/NIAMS NIH HHS/United States
- R21 AI087990/AI/NIAID NIH HHS/United States
- KL2 RR024132/RR/NCRR NIH HHS/United States
- T32 AR007465/AR/NIAMS NIH HHS/United States
- AI095608/AI/NIAID NIH HHS/United States
- P30 CA016520/CA/NCI NIH HHS/United States
- R01 AI097333/AI/NIAID NIH HHS/United States
- AI095466/AI/NIAID NIH HHS/United States
- F32-AI085828/AI/NIAID NIH HHS/United States
- AI097333/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

