Bioreduction of Cr(VI) by alkaliphilic Bacillus subtilis and interaction of the membrane groups
- PMID: 23961119
- PMCID: PMC3730869
- DOI: 10.1016/j.sjbs.2010.12.003
Bioreduction of Cr(VI) by alkaliphilic Bacillus subtilis and interaction of the membrane groups
Abstract
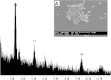
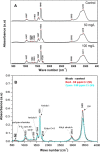
Detoxification of Cr(VI) under alkaline pH requires attention due to the alkaline nature of many effluents. An alkaliphilic gram-positive Bacillus subtilis isolated from tannery effluent contaminated soil was found to grow and reduce Cr(VI) up to 100% at an alkaline pH 9. Decrease in pH to acidic range with growth of the bacterium signified the role played by metabolites (organic acids) in chromium resistance and reduction mechanism. The XPS and FT-IR spectra confirmed the reduction of Cr(VI) by bacteria into +3 oxidation state. Chromate reductase assay indicated that the reduction was mediated by constitutive membrane bound enzymes. The kinetics of Cr(VI) reduction activity derived using the monod equation proved (K s = 0.00032) high affinity of the organism to the metal. This study thus helped to localize the reduction activity at subcellular level in a chromium resistant alkaliphilic Bacillus sp.
Keywords: 16S rRNA identification; Bacillus sp.; Chromate reductase; Cr(VI) reduction; Membrane bound proteins.
Figures





Similar articles
-
In vitro Cr(VI) reduction by cell-free extracts of chromate-reducing bacteria isolated from tannery effluent irrigated soil.Environ Sci Pollut Res Int. 2013 Mar;20(3):1661-74. doi: 10.1007/s11356-012-1178-4. Epub 2012 Sep 15. Environ Sci Pollut Res Int. 2013. PMID: 22983604
-
Hexavalent chromium reduction by chromate-resistant haloalkaliphilic Halomonas sp. M-Cr newly isolated from tannery effluent.Biotechnol Biotechnol Equip. 2014 Jul 4;28(4):659-667. doi: 10.1080/13102818.2014.937092. Epub 2014 Oct 17. Biotechnol Biotechnol Equip. 2014. PMID: 26740769 Free PMC article.
-
Chromate reduction by chromium-resistant bacteria isolated from soils contaminated with dichromate.J Environ Qual. 2003 Jul-Aug;32(4):1228-33. doi: 10.2134/jeq2003.1228. J Environ Qual. 2003. PMID: 12931876
-
Bioreduction and biosorption of Cr(VI) by a novel Bacillus sp. CRB-B1 strain.J Hazard Mater. 2020 Mar 15;386:121628. doi: 10.1016/j.jhazmat.2019.121628. Epub 2019 Nov 6. J Hazard Mater. 2020. PMID: 31744729
-
Genetic correlation between chromium resistance and reduction in Bacillus brevis isolated from tannery effluent.J Appl Microbiol. 2009 Nov;107(5):1425-32. doi: 10.1111/j.1365-2672.2009.04326.x. Epub 2009 Apr 22. J Appl Microbiol. 2009. PMID: 19426270
Cited by
-
A Comprehensive Review of the Current Progress of Chromium Removal Methods from Aqueous Solution.Toxics. 2023 Mar 8;11(3):252. doi: 10.3390/toxics11030252. Toxics. 2023. PMID: 36977017 Free PMC article. Review.
-
Bioreduction and immobilization of hexavalent chromium by the extremely acidophilic Fe(III)-reducing bacterium Acidocella aromatica strain PFBC.Extremophiles. 2015 Mar;19(2):495-503. doi: 10.1007/s00792-015-0733-6. Epub 2015 Feb 5. Extremophiles. 2015. PMID: 25651881
-
Transcriptome Analysis Reveals Cr(VI) Adaptation Mechanisms in Klebsiella sp. Strain AqSCr.Front Microbiol. 2021 May 27;12:656589. doi: 10.3389/fmicb.2021.656589. eCollection 2021. Front Microbiol. 2021. PMID: 34122372 Free PMC article.
-
Biotransformation of Chromium (VI) via a Reductant Activity from the Fungal Strain Purpureocillium lilacinum.J Fungi (Basel). 2021 Nov 29;7(12):1022. doi: 10.3390/jof7121022. J Fungi (Basel). 2021. PMID: 34947004 Free PMC article.
-
Hexavalent chromium removal by a novel Serratia proteamaculans isolated from the bank of Sebou River (Morocco).Environ Sci Pollut Res Int. 2014 Feb;21(4):3060-72. doi: 10.1007/s11356-013-2249-x. Epub 2013 Nov 6. Environ Sci Pollut Res Int. 2014. PMID: 24194414
References
-
- Aravindhan R., Madhan B., Raghava Rao J., Unni Nair B., Ramasami T. Bioaccumulation of chromium from the tannery waste water: an approach for chrome recovery and reuse. Environ. Sci. Technol. 2004;38:300–306. - PubMed
-
- Asatiani N.V., Abuladze M.K., Kartvelishvili T.M., Bakradze N.G., Sapojnikova N.A., Tsibakhashvili N.Y., Tabatadze L.V., Lejava L.V., Asanishvili L.L., Holman H.Y. Effect of Cr(VI) action on Arthrobacter oxydans. Curr. Microbiol. 2004;49:321–326. - PubMed
-
- Asenjo J.A. vol. 9. CRC Press; New York: 1990. (Separation Processes in Biotechnology).
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

