Identifying frankincense impact by biochemical analysis and histological examination on rats
- PMID: 23961123
- PMCID: PMC3730859
- DOI: 10.1016/j.sjbs.2010.10.005
Identifying frankincense impact by biochemical analysis and histological examination on rats
Abstract
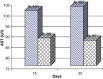
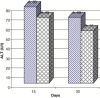
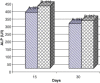
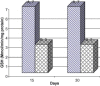
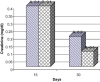
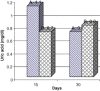
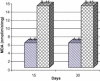
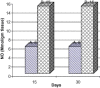
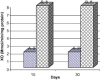
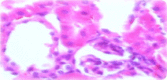
Frankincense (Gum Olibanum), made from resins of Burseraceae family, grows in Somalia, India and Yemen. Many years ago the oldest doctors used this plant for treatment of many diseases. This study identifies frankincense impact by biochemical analysis and histological examination on rats. In this study, forty male Wister Albino rats weighing 70-100 g were maintained in clean cages. The rats were divided into 2 groups, each group contained 20 rats. Frankincense extract was prepared by heating distilled water (400 ml) to 80 °C and soaking 20 g of herbs for about 60 min. After cooking at room temperature the dose was given orally through special drinking bottles daily. The first group acted as control drinking water. The second group served as treated group and was given frankincense in the drinking water during the whole duration of the experiment. After 15 and 30 days of treatment, the rats were anesthetized with ether, and blood was collected from the livers and kidneys; some biochemical analyses were performed including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and non-bilirubin, urea, uric acid, and creatinine. Rats were killed by cervical decapitation of livers and kidneys. Each group was divided into 2 parts. The first part was used for the determination of glutathione (GSH), glucose 6 phosphate dehydrogenase (G6PDH), xanthine oxidase (XO), malonyldealdehyde (MDA), nitric oxide (NO), and xanthine oxidase (XO). The second part of livers and kidneys was kept in formalin solution (10%) and stained by Hematoxylin and Eosin (H & E), to be used for histological examination. I demonstrated in the biochemical analysis in the serum, tissue and histological examination, different impact between group (B) and group (A), and that frankincense is not absolutely safe and that precautions must be taken during it's us as a traditional medicine and that increase the awareness with safety and health hazards of many other traditional medicine is critically needed.
Keywords: AST; Boswella Serrata; Frankincense; GSH; Histology; MDA.
Figures










References
-
- Bancroft J.D., Stevens A. Churchill, Livingston; Edin burgh, London, Melbourne and New York: 1996. Theory and practice of histological technique, (fourth ed.) pp. 50–56.
-
- Bentler E., Duran O., Mikus K.B. Improved method for determination of blood glutathione. J. Lab. Clin. Med. 1963;61:882. - PubMed
-
- Bergmeyer H.U., Bernt E., Grassl M., Michel G. Glucose-6-phosphate dehydrogenase. In: Bergmeyer U.H., editor. Methods of Enzymatic Analysis. Verlag Chemie Weinheim; Academic Press, New York: 1974. pp. 458–459.
-
- Bergmeyer H.U., Scheibe P., Wahlefeld W.W. Optimization of methods for aspartate amino transferase and alanine amino transferase. Clin. Chem. 1978;24(1):58–73. - PubMed
-
- Buege J.A., Aust S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–315. - PubMed
Further reading
-
- Zhu N., Kikuzaki H., Sheng S., Sang S., Rafi M.M., Wang M., Nakatani N., Dipaola R.S., Rosan R.T., No C.T. Furanosesquiteroenoids of commiphora myrrha. J. Nat. Prod. 2001;64(11):1460–1462. - PubMed
LinkOut - more resources
Full Text Sources

