Fetal endocrinology
- PMID: 23961471
- PMCID: PMC3743355
- DOI: 10.4103/2230-8210.113722
Fetal endocrinology
Abstract
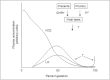
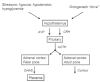
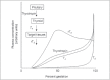
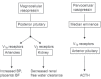
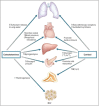
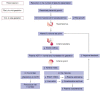
Successful outcome of pregnancy depends upon genetic, cellular, and hormonal interactions, which lead to implantation, placentation, embryonic, and fetal development, parturition and fetal adaptation to extrauterine life. The fetal endocrine system commences development early in gestation and plays a modulating role on the various physiological organ systems and prepares the fetus for life after birth. Our current article provides an overview of the current knowledge of several aspects of this vast field of fetal endocrinology and the role of endocrine system on transition to extrauterine life. We also provide an insight into fetal endocrine adaptations pertinent to various clinically important situations like placental insufficiency and maternal malnutrition.
Keywords: Endocrinology; fetal; hypothalamic; pituitary.
Conflict of interest statement
Figures


References
-
- Grumbach MM, Gluckman PD. The human fetal hypothalamus and pituitary gland: The maturation of neuroendocrine mechanisms controlling secretion of fetal pituitary growth hormone, prolactin, gonadotropins, adrenocorticotropin-related peptides, and thyrotropin. In: Tulchinsky D, Little AB, editors. Maternal Fetal Endocrinology. 2nd ed. Philadelphia: WB Saunders; 1994. pp. 193–261.
-
- Wu Y, He Z, Zhang L, Jiang H, Zhang W. Ontogeny of immunoreactive Lh and Fsh cells in relation to early ovarian differentiation and development in protogynous hermaphroditic ricefield Eel Monopterus albus. Biol Reprod. 2012;86:93. - PubMed
-
- Pepe GJ, Albrecht ED. Regulation of the primate fetal adrenal cortex. Endocr Rev. 1990;11:151–76. - PubMed
-
- Erickson RP, Blecher SR. Genetics of sex determination and differentiation. In: Polin RA, Fox WW, Abman SH, editors. Fetal and Neonatal Physiology. 3rd ed. Philadelphia: WB Saunders; 2004. pp. 1935–41.
-
- Lee MM. Molecular genetic control of sex differentiation. In: Pescovitz OH, Eugster EA, editors. Pediatric Endocrinology. Philadelphia: Lippincott Williams and Wilkins; 2004. pp. 231–42.
LinkOut - more resources
Full Text Sources
Other Literature Sources

