Access to the protoilludane core by gold-catalyzed allene-vinylcyclopropane cycloisomerization
- PMID: 23962171
- PMCID: PMC3774677
- DOI: 10.1021/ol402188b
Access to the protoilludane core by gold-catalyzed allene-vinylcyclopropane cycloisomerization
Erratum in
- Org Lett. 2013 Oct 4;15(19):5146
Abstract
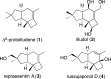
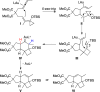
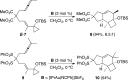
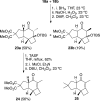
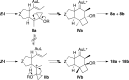
Gold(I)-catalyzed allene-vinylcyclopropane cycloisomerization leads to the tricyclic framework of the protoilludanes in a single step by a reaction that involves a cyclopropane ring expansion and a Prins cyclization.
Figures
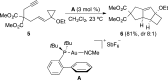



References
-
- Tanasova M.; Sturla S. J. Chem. Rev. 2012, 112, 3578–3610. - PubMed
-
-
For a review, see:
- Siengalewicz P.; Mulzer J.; Rinner U. Eur. J. Org. Chem. 2011, 7041–7055.
- See also: Schwartz B. D.; Matoušová E.; White R.; Banwell M. G.; Willis A. C. Org. Lett. 2013, 15, 1934–1937. - PubMed
-
-
- Hansen T. V.; Stenstrøm Y. Naturally Occuring Cyclobutanes. Organic Synthesis: Theory and Applications; Hudlicky J. A. I.Elsevier Science Ltd.: Oxford, 2001; pp 1–39.
-
-
For total synthesis, see:
- Furukawa J.; Morisaki N.; Kobayashi H.; Iwasaki S.; Nozoe S.; Okuda S. Chem. Pharm. Bull. 1985, 33, 440–443.
- Oppolzer W.; Nakao A. Tetrahedron Lett. 1986, 27, 5471–5474.
-
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

