Influenza virus A M2 protein generates negative Gaussian membrane curvature necessary for budding and scission
- PMID: 23962302
- PMCID: PMC4000230
- DOI: 10.1021/ja400146z
Influenza virus A M2 protein generates negative Gaussian membrane curvature necessary for budding and scission
Abstract
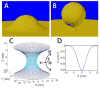
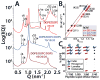
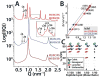
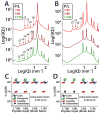
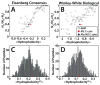
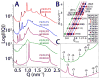
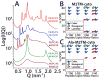
The M2 protein is a multifunctional protein, which plays several roles in the replication cycle of the influenza A virus. Here we focus on its ability to promote budding of the mature virus from the cell surface. Using high-resolution small-angle X-ray scattering we show that M2 can restructure lipid membranes into bicontinuous cubic phases which are rich in negative Gaussian curvature (NGC). The active generation of negative Gaussian membrane curvature by M2 is essential to influenza virus budding. M2 has been observed to colocalize with the region of high NGC at the neck of a bud. The structural requirements for scission are even more stringent than those for budding, as the neck must be considerably smaller than the virus during 'pinch off'. Consistent with this, the amount of NGC in the induced cubic phases suggests that M2 proteins can generate high curvatures comparable to those on a neck with size 10× smaller than a spherical influenza virus. Similar experiments on variant proteins containing different M2 domains show that the cytoplasmic amphipathic helix is necessary and sufficient for NGC generation. Mutations to the helix which reduce its amphiphilicity and are known to diminish budding attenuated NGC generation. An M2 construct comprising the membrane interactive domains, the transmembrane helix and the cytoplasmic helix, displayed enhanced ability to generate NGC, suggesting that other domains cooperatively promote membrane curvature. These studies establish the importance of M2-induced NGC during budding and suggest that antagonizing this curvature is a viable anti-influenza strategy.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

