Systemic sclerosis sera affect fibrillin-1 deposition by dermal blood microvascular endothelial cells: therapeutic implications of cyclophosphamide
- PMID: 23962393
- PMCID: PMC3978697
- DOI: 10.1186/ar4270
Systemic sclerosis sera affect fibrillin-1 deposition by dermal blood microvascular endothelial cells: therapeutic implications of cyclophosphamide
Abstract
Introduction: Systemic sclerosis (SSc) is a connective tissue disorder characterized by endothelial cell injury, autoimmunity and fibrosis. The following three fibrillin-1 alterations have been reported in SSc. (1) Fibrillin-1 microfibrils are disorganized in SSc dermis. (2) Fibrillin-1 microfibrils produced by SSc fibroblasts are unstable. (3) Mutations in the FBN1 gene and anti-fibrillin-1 autoantibodies have been reported in SSc. Fibrillin-1 microfibrils, which are abundantly produced by blood and lymphatic microvascular endothelial cells (B-MVECs and Ly-MVECs, respectively), sequester in the extracellular matrix the latent form of the potent profibrotic cytokine transforming growth factor β (TGF-β). In the present study, we evaluated the effects of SSc sera on the deposition of fibrillin-1 and microfibril-associated glycoprotein 1 (MAGP-1) and the expression of focal adhesion molecules by dermal B-MVECs and Ly-MVECs.
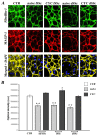
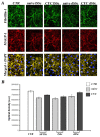
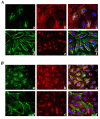
Methods: Dermal B-MVECs and Ly-MVECs were challenged with sera from SSc patients who were treatment-naïve or under cyclophosphamide (CYC) treatment and with sera from healthy controls. Fibrillin-1/MAGP-1 synthesis and deposition and the expression of αvβ₃ integrin/phosphorylated focal adhesion kinase and vinculin/actin were evaluated by immunofluorescence and quantified by morphometric analysis.
Results: Fibrillin-1 and MAGP-1 colocalized in all experimental conditions, forming a honeycomb pattern in B-MVECs and a dense mesh of short segments in Ly-MVECs. In B-MVECs, fibrillin-1/MAGP-1 production and αvβ₃ integrin expression significantly decreased upon challenge with sera from naïve SSc patients compared with healthy controls. Upon challenge of B-MVECs with sera from CYC-treated SSc patients, fibrillin-1/MAGP-1 and αvβ₃ integrin levels were comparable to those of cells treated with healthy sera. Ly-MVECs challenged with SSc sera did not differ from those treated with healthy control sera in the expression of any of the molecules assayed.
Conclusions: Because of the critical role of fibrillin-1 in sequestering the latent form of TGF-β in the extracellular matrix, its decreased deposition by B-MVECs challenged with SSc sera might contribute to dermal fibrosis. In SSc, CYC treatment might limit fibrosis through the maintenance of physiologic fibrillin-1 synthesis and deposition by B-MVECs.
Figures

Similar articles
-
Mutant fibrillin 1 from tight skin mice increases extracellular matrix incorporation of microfibril-associated glycoprotein 2 and type I collagen.Arthritis Rheum. 2004 Mar;50(3):915-26. doi: 10.1002/art.20053. Arthritis Rheum. 2004. PMID: 15022335
-
Human microvascular lymphatic and blood endothelial cells produce fibrillin: deposition patterns and quantitative analysis.J Anat. 2010 Dec;217(6):705-14. doi: 10.1111/j.1469-7580.2010.01306.x. Epub 2010 Oct 11. J Anat. 2010. PMID: 21039476 Free PMC article.
-
Abnormalities in fibrillin 1-containing microfibrils in dermal fibroblast cultures from patients with systemic sclerosis (scleroderma).Arthritis Rheum. 2001 Aug;44(8):1855-64. doi: 10.1002/1529-0131(200108)44:8<1855::AID-ART324>3.0.CO;2-Q. Arthritis Rheum. 2001. PMID: 11508439
-
Fibrillin in Marfan syndrome and tight skin mice provides new insights into transforming growth factor-beta regulation and systemic sclerosis.Curr Opin Rheumatol. 2006 Nov;18(6):582-7. doi: 10.1097/01.bor.0000245719.64393.57. Curr Opin Rheumatol. 2006. PMID: 17053502 Review.
-
Endothelial dysfunction in systemic sclerosis.Curr Opin Rheumatol. 2014 Nov;26(6):615-20. doi: 10.1097/BOR.0000000000000112. Curr Opin Rheumatol. 2014. PMID: 25191994 Review.
Cited by
-
Anti-vinculin antibodies as a novel biomarker in Egyptian patients with systemic sclerosis.Clin Rheumatol. 2022 Nov;41(11):3401-3409. doi: 10.1007/s10067-022-06301-0. Epub 2022 Jul 25. Clin Rheumatol. 2022. PMID: 35876914 Free PMC article.
-
Potential Effect of Cyclophosphamide on Bleb Survival in Five Patients with Multiple Sclerosis Who Underwent Glaucoma Surgery.Ophthalmol Ther. 2018 Dec;7(2):431-436. doi: 10.1007/s40123-018-0133-y. Epub 2018 Jun 7. Ophthalmol Ther. 2018. PMID: 29882152 Free PMC article.
-
Mass spectrometry-based proteomics identify novel serum osteoarthritis biomarkers.Arthritis Res Ther. 2022 May 23;24(1):120. doi: 10.1186/s13075-022-02801-1. Arthritis Res Ther. 2022. PMID: 35606786 Free PMC article.
-
Systemic Sclerosis Sera Impair Angiogenic Performance of Dermal Microvascular Endothelial Cells: Therapeutic Implications of Cyclophosphamide.PLoS One. 2015 Jun 15;10(6):e0130166. doi: 10.1371/journal.pone.0130166. eCollection 2015. PLoS One. 2015. PMID: 26076019 Free PMC article.
-
Identification of lncRNA-miRNA-mRNA networks in circulating exosomes as potential biomarkers for systemic sclerosis.Front Med (Lausanne). 2023 Feb 16;10:1111812. doi: 10.3389/fmed.2023.1111812. eCollection 2023. Front Med (Lausanne). 2023. PMID: 36873898 Free PMC article.
References
-
- Solito R, Alessandrini C, Fruschelli M, Pucci AM, Gerli R. An immunological correlation between the anchoring filaments of initial lymph vessels and the neighboring elastic fibers: a unified morphofunctional concept. Lymphology. 1997;15:194–202. - PubMed
-
- Gerli R, Ibba L, Fruschelli C. A fibrillar elastic apparatus around human lymph capillaries. Anat Embryol (Berl) 1990;15:281–286. - PubMed
-
- Rossi A, Weber E, Sacchi G, Maestrini D, Di Cintio F, Gerli R. Mechanotrasduction in lymphatic endothelial cells. Lymphology. 2007;15:102–113. - PubMed
-
- Robinson PN, Arteaga-Solis E, Baldock C, Collod-Béroud G, Booms P, De Paepe A, Dietz HC, Guo G, Handford PA, Judge DP, Kielty CM, Loeys B, Milewicz DM, Ney A, Ramirez F, Reinhardt DP, Tiedemann K, Whiteman P, Godfrey M. The molecular genetics of Marfan syndrome and related disorders. J Med Genet. 2006;15:769–787. doi: 10.1136/jmg.2005.039669. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

