Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: two-year efficacy and safety findings from AMPLE trial
- PMID: 23962455
- PMCID: PMC3888617
- DOI: 10.1136/annrheumdis-2013-203843
Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: two-year efficacy and safety findings from AMPLE trial
Abstract
Objectives: To compare over 2 years the safety, efficacy and radiographic outcomes of subcutaneous abatacept versus adalimumab, in combination with methotrexate (MTX), in patients with rheumatoid arthritis (RA).
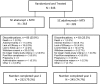
Methods: AMPLE is a phase IIIb, 2-year, randomised, investigator-blinded study with a 1-year primary endpoint. Biologic-naive patients with active RA and an inadequate response to MTX were randomised to 125 mg abatacept weekly or 40 mg adalimumab bi-weekly, both with a stable dose of MTX.
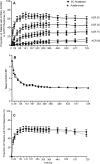
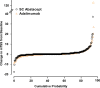
Results: Of 646 patients randomised, 79.2% abatacept and 74.7% adalimumab patients completed year 2. At year 2, efficacy outcomes, including radiographic, remained comparable between groups and with year 1 results. The American College Rheumatology 20, 50 and 70 responses at year 2 were 59.7%, 44.7% and 31.1% for abatacept and 60.1%, 46.6% and 29.3% for adalimumab. There were similar rates of adverse events (AEs) and serious adverse events (SAEs). More serious infections occurred with adalimumab (3.8% vs 5.8%) including two cases of tuberculosis with adalimumab. There were fewer discontinuations due to AEs (3.8% vs 9.5%), SAEs (1.6% vs 4.9%) and serious infections (0/12 vs 9/19 patients) in the abatacept group. Injection site reactions (ISRs) occurred less frequently with abatacept (4.1% vs 10.4%).
Conclusions: Through 2 years of blinded treatment in this first head-to-head study between biologic disease-modifying antirheumatic drugs in RA patients with an inadequate response to MTX, subcutaneous abatacept and adalimumab were similarly efficacious based on clinical, functional and radiographic outcomes. Overall, AE frequency was similar in both groups but there were less discontinuations due to AEs, SAEs, serious infections and fewer local ISRs with abatacept.
Trial registration: ClinicalTrials.gov NCT00929864.
Keywords: DMARDs (Biologic); Methotrexate; Rheumatoid Arthritis.
Figures

References
-
- Knevel R, Schoels M, Huizinga TW, et al. Current evidence for a strategic approach to the management of rheumatoid arthritis with disease-modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2010;69:987–94 - PubMed
-
- Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26–37 - PubMed
-
- Klareskog L, van der HD, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 2004;363:675–81 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

