Identification of Borrelia burgdorferi ospC genotypes in canine tissue following tick infestation: implications for Lyme disease vaccine and diagnostic assay design
- PMID: 23962611
- PMCID: PMC3872846
- DOI: 10.1016/j.tvjl.2013.07.019
Identification of Borrelia burgdorferi ospC genotypes in canine tissue following tick infestation: implications for Lyme disease vaccine and diagnostic assay design
Abstract
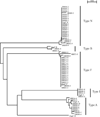
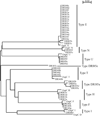
In endemic regions, Lyme disease is a potential health threat to dogs. Canine Lyme disease manifests with arthritis-induced lameness, anorexia, fever, lethargy, lymphadenopathy and, in some cases, fatal glomerulonephritis. A recent study revealed that the regional mean for the percentage of seropositive dogs in the north-east of the USA is 11.6%. The outer surface protein C (OspC) of Lyme disease spirochetes is an important virulence factor required for the establishment of infection in mammals. It is a leading candidate in human and canine Lyme disease vaccine development efforts. Over 30 distinct ospC phyletic types have been defined. It has been hypothesized that ospC genotype may influence mammalian host range. In this study, Ixodes scapularis ticks collected from the field in Rhode Island were assessed for infection with B. burgdorferi. Ticks were fed on purpose bred beagles to repletion and infection of the dogs was assessed through serology and PCR. Tissue biopsies (n=2) were collected from each dog 49 days post-tick infestation (dpi) and the ospC genotype of the infecting strains determined by direct PCR of DNA extracted from tissue or by PCR after cultivation of spirochetes from biopsy samples. The dominant ospC types associated with B. burgdorferi canine infections differed from those associated with human infection, indicating a relationship between ospC sequence and preferred host range. Knowledge of the most common ospC genotypes associated specifically with infection of dogs will facilitate the rational design of OspC-based canine Lyme disease vaccines and diagnostic assays.
Keywords: Borrelia burgdorferi; Canine; Lyme disease; OspC; Ticks; Vaccine.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Intellectual property developed by Drs Marconi and Earnhart as an extension of this study is owned and is being patented by Virginia Commonwealth University. Zoetis has licensed this intellectual property and has contributed in part to funding this research.
Figures
Similar articles
-
Bacterin that induces anti-OspA and anti-OspC borreliacidal antibodies provides a high level of protection against canine Lyme disease.Clin Vaccine Immunol. 2009 Feb;16(2):253-9. doi: 10.1128/CVI.00373-08. Epub 2008 Dec 3. Clin Vaccine Immunol. 2009. PMID: 19052162 Free PMC article.
-
Immunization with a recombinant subunit OspA vaccine markedly impacts the rate of newly acquired Borrelia burgdorferi infections in client-owned dogs living in a coastal community in Maine, USA.Parasit Vectors. 2015 Feb 10;8:92. doi: 10.1186/s13071-015-0676-x. Parasit Vectors. 2015. PMID: 25890386 Free PMC article.
-
A fluorescent bead-based multiplex assay for the simultaneous detection of antibodies to B. burgdorferi outer surface proteins in canine serum.Vet Immunol Immunopathol. 2011 Apr 15;140(3-4):190-8. doi: 10.1016/j.vetimm.2010.12.003. Epub 2010 Dec 10. Vet Immunol Immunopathol. 2011. PMID: 21208663
-
The role of Ixodes scapularis, Borrelia burgdorferi and wildlife hosts in Lyme disease prevalence: A quantitative review.Ticks Tick Borne Dis. 2018 Jul;9(5):1103-1114. doi: 10.1016/j.ttbdis.2018.04.006. Epub 2018 Apr 16. Ticks Tick Borne Dis. 2018. PMID: 29680260 Review.
-
Diversity of the Lyme Disease Spirochetes and its Influence on Immune Responses to Infection and Vaccination.Vet Clin North Am Small Anim Pract. 2019 Jul;49(4):671-686. doi: 10.1016/j.cvsm.2019.02.007. Epub 2019 Apr 6. Vet Clin North Am Small Anim Pract. 2019. PMID: 30967254 Free PMC article. Review.
Cited by
-
Protection against Borreliella burgdorferi infection mediated by a synthetically engineered DNA vaccine.Hum Vaccin Immunother. 2020 Sep 1;16(9):2114-2122. doi: 10.1080/21645515.2020.1789408. Epub 2020 Aug 12. Hum Vaccin Immunother. 2020. PMID: 32783701 Free PMC article.
-
A Bayesian spatio-temporal model for forecasting the prevalence of antibodies to Borrelia burgdorferi, causative agent of Lyme disease, in domestic dogs within the contiguous United States.PLoS One. 2017 May 4;12(5):e0174428. doi: 10.1371/journal.pone.0174428. eCollection 2017. PLoS One. 2017. PMID: 28472096 Free PMC article.
-
Human and Veterinary Vaccines for Lyme Disease.Curr Issues Mol Biol. 2021;42:191-222. doi: 10.21775/cimb.042.191. Epub 2020 Dec 8. Curr Issues Mol Biol. 2021. PMID: 33289681 Free PMC article.
-
Borrelia burgdorferi Keeps Moving and Carries on: A Review of Borrelial Dissemination and Invasion.Front Immunol. 2017 Feb 21;8:114. doi: 10.3389/fimmu.2017.00114. eCollection 2017. Front Immunol. 2017. PMID: 28270812 Free PMC article. Review.
-
Characterization of recombinant OspA in two different Borrelia vaccines with respect to immunological response and its relationship to functional parameters.BMC Vet Res. 2018 Oct 16;14(1):312. doi: 10.1186/s12917-018-1625-7. BMC Vet Res. 2018. PMID: 30326885 Free PMC article.
References
-
- Alghaferi MY, Anderson JM, Park J, Auwaerter PG, Aucott JN, Norris DE, Dumler JS. Borrelia burgdorferi ospC heterogeneity among human and murine isolates from a defined region of northern Maryland and southern Pennsylvania: Lack of correlation with invasive and noninvasive genotypes. Journal of Clinical Microbiology. 2005;43:1879–1884. - PMC - PubMed
-
- Benach JL, Bosler EM, Hanrahan JP, Coleman JL, Habicht GS, Bast TF, Cameron DJ, Ziegler JL, Barbour AG, Burgdorfer W, et al. Spirochetes isolated from the blood of two patients with Lyme disease. New England Journal of Medicine. 1983;308:740–742. - PubMed
-
- Baranton G, Postic D, Saint Girons I, Boerlin P, Piffaretti J-C, Assous M, Grimont PAD. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., group VS461 associated with Lyme borreliosis. International Journal of Systematic Bacteriology. 1992;42:378–383. - PubMed
-
- Bowman D, Little SE, Lorentzen L, Shields J, Sullivan MP, Carlin EP. Prevalence and geographic distribution of Dirofilaria immitis, Borrelia burgdorferi, Ehrlichia canis and in dogs in the United States: Results of a national clinic-based serologic survey. Veterinary Parasitology. 2009;160:138–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

