Chronic exercise downregulates myocardial myoglobin and attenuates nitrite reductase capacity during ischemia-reperfusion
- PMID: 23962643
- PMCID: PMC3800246
- DOI: 10.1016/j.yjmcc.2013.08.002
Chronic exercise downregulates myocardial myoglobin and attenuates nitrite reductase capacity during ischemia-reperfusion
Abstract
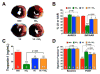
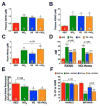
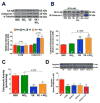
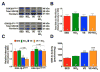
The infarct sparing effects of exercise are evident following both long-term and short-term training regimens. Here we compared the infarct-lowering effects of nitrite therapy, voluntary exercise, and the combination of both following myocardial ischemia-reperfusion (MI/R) injury. We also compared the degree to which each strategy increased cardiac nitrite levels, as well as the effects of each strategy on the nitrite reductase activity of the heart. Mice subjected to voluntary wheel running (VE) for 4weeks displayed an 18% reduction in infarct size when compared to sedentary mice, whereas mice administered nitrite therapy (25mg/L in drinking water) showed a 53% decrease. However, the combination of VE and nitrite exhibited no further protection than VE alone. Although the VE and nitrite therapy mice showed similar nitrite levels in the heart, cardiac nitrite reductase activity was significantly reduced in the VE mice. Additionally, the cardiac protein expression of myoglobin, a known nitrite reductase, was also reduced after VE. Further studies revealed that cardiac NFAT activity was lower after VE due to a decrease in calcineurin activity and an increase in GSK3β activity. These data suggest that VE downregulates cardiac myoglobin levels by inhibiting calcineurin/NFAT signaling. Additionally, these results suggest that the modest infarct sparing effects of VE are the result of a decrease in the hearts ability to reduce nitrite to nitric oxide during MI/R.
Keywords: Calcineurin/NFAT signaling; Exercise; Myocardial ischemia–reperfusion; Myoglobin; Nitric oxide; Nitrite.
© 2013.
Figures


Similar articles
-
Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury.Proc Natl Acad Sci U S A. 2008 Jul 22;105(29):10256-61. doi: 10.1073/pnas.0801336105. Epub 2008 Jul 16. Proc Natl Acad Sci U S A. 2008. PMID: 18632562 Free PMC article.
-
A highlight of myoglobin diversity: the nitrite reductase activity during myocardial ischemia-reperfusion.Nitric Oxide. 2010 Feb 15;22(2):75-82. doi: 10.1016/j.niox.2009.10.003. Epub 2009 Oct 29. Nitric Oxide. 2010. PMID: 19836457 Review.
-
Exercise protects against myocardial ischemia-reperfusion injury via stimulation of β(3)-adrenergic receptors and increased nitric oxide signaling: role of nitrite and nitrosothiols.Circ Res. 2011 Jun 10;108(12):1448-58. doi: 10.1161/CIRCRESAHA.111.241117. Epub 2011 Apr 28. Circ Res. 2011. PMID: 21527738 Free PMC article.
-
Assessment of the functional diversity of human myoglobin.Nitric Oxide. 2012 May 15;26(4):211-6. doi: 10.1016/j.niox.2012.03.001. Epub 2012 Mar 8. Nitric Oxide. 2012. PMID: 22425779
-
The emerging role of nitrite as an endogenous modulator and therapeutic agent of cardiovascular function.Curr Med Chem. 2010;17(18):1915-25. doi: 10.2174/092986710791163948. Curr Med Chem. 2010. PMID: 20377513 Review.
Cited by
-
Sustained release nitrite therapy results in myocardial protection in a porcine model of metabolic syndrome with peripheral vascular disease.Am J Physiol Heart Circ Physiol. 2015 Jul 15;309(2):H305-17. doi: 10.1152/ajpheart.00163.2015. Epub 2015 May 8. Am J Physiol Heart Circ Physiol. 2015. PMID: 25957218 Free PMC article.
-
Exercise training and experimental myocardial ischemia and reperfusion: A systematic review and meta-analysis.Int J Cardiol Heart Vasc. 2023 Apr 29;46:101214. doi: 10.1016/j.ijcha.2023.101214. eCollection 2023 Jun. Int J Cardiol Heart Vasc. 2023. PMID: 37181278 Free PMC article. Review.
-
The Impact of Normobaric Hypoxia and Intermittent Hypoxic Training on Cardiac Biomarkers in Endurance Athletes: A Pilot Study.Int J Mol Sci. 2024 Apr 23;25(9):4584. doi: 10.3390/ijms25094584. Int J Mol Sci. 2024. PMID: 38731803 Free PMC article.
-
Therapeutic potential of sustained-release sodium nitrite for critical limb ischemia in the setting of metabolic syndrome.Am J Physiol Heart Circ Physiol. 2015 Jul 1;309(1):H82-92. doi: 10.1152/ajpheart.00115.2015. Epub 2015 Apr 24. Am J Physiol Heart Circ Physiol. 2015. PMID: 25910804 Free PMC article.
-
Angiotensin type 2-receptor (AT2R) activation induces hypotension in apolipoprotein E-deficient mice by activating peroxisome proliferator-activated receptor-γ.Am J Cardiovasc Dis. 2016 Sep 15;6(3):118-28. eCollection 2016. Am J Cardiovasc Dis. 2016. PMID: 27679746 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

