Somatostatin receptor type 2-based reporter expression after plasmid-based in vivo gene delivery to non-small cell lung cancer
- PMID: 23962694
- PMCID: PMC4103180
Somatostatin receptor type 2-based reporter expression after plasmid-based in vivo gene delivery to non-small cell lung cancer
Abstract
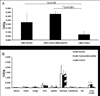
Plasmids tend to have much lower expression than viruses. Gene expression after systemic administration of plasmid vectors has not been assessed using somatostatin receptor type 2 (SSTR2)-based reporters. The purpose of this work was to identify gene expression in non-small cell lung cancer (NSCLC) after systemic liposomal nanoparticle delivery of plasmid containing SSTR2-based reporter gene. In vitro, Western blotting was performed after transient transfection with the plasmid cytomegalovirus (CMV)-SSTR2, CMV-TUSC2-IRES-SSTR2, or CMV-TUSC2. SSTR2 is the reporter gene, and TUSC2 is a therapeutic gene. Mice with A549 NSCLC lung tumors were injected intravenously with CMV-SSTR2, CMV-TUSC2-IRES-SSTR2, or CMV-TUSC2 plasmids in DOTAP:cholesterol-liposomal nanoparticles. Two days later, mice were injected intravenously with 111In-octreotide. The next day, biodistribution was performed. The experiment was repeated including single-photon emission computed tomography/computed tomography (SPECT/CT). Immunohistochemistry was performed. In vitro, SSTR2 expression was similar in cells transfected with CMV-SSTR2 or CMV-TUSC2-IRES-SSTR2. TUSC2 expression was similar in cells transfected with CMV-TUSC2 or CMV-TUSC2-SSTR2. Biodistribution demonstrated significantly greater 111In-octreotide uptake in tumors from mice injected with CMV-TUSC2-IRES-SSTR2 or CMV-SSTR2 than the control plasmid, CMV-TUSC2 (p < .05). Gamma-camera and SPECT/CT imaging illustrated SSTR2 expression in tumors in mice injected with CMV-TUSC2-IRES-SSTR2 or CMV-SSTR2 versus background with control plasmid. Immunohistochemistry corresponded with imaging. SSTR2-based reporter imaging can visualize gene expression in lung tumors after systemic liposomal nanoparticle delivery of plasmid containing SSTR2-based reporter gene or SSTR2 linked to a second therapeutic gene, such as TUSC2.
Conflict of interest statement
Conflict of Interest: JAR: Consultancy, grants, stock: Genprex, Inc.; Patents pending and issued: TUSC2/FUS1 NST: Novel DNA:liposome complexes for increased systemic delivery and gene expression. United States Letters Patent No. 6,413,544 B1 issued July 2, 2002. United States Patent No. 6,770,291 B2 issued August 3, 2004. International Publication Number: WO 98/07408. The institution holds patents regarding using SSTR2-based reporters.
Figures






Similar articles
-
Somatostatin receptor type 2 as a radiotheranostic PET reporter gene for oncologic interventions.Theranostics. 2018 May 23;8(12):3380-3391. doi: 10.7150/thno.24017. eCollection 2018. Theranostics. 2018. PMID: 29930736 Free PMC article.
-
SSTR2-based reporters for assessing gene transfer into non-small cell lung cancer: evaluation using an intrathoracic mouse model.Hum Gene Ther. 2011 Jan;22(1):55-64. doi: 10.1089/hum.2010.109. Epub 2010 Dec 6. Hum Gene Ther. 2011. PMID: 20653396 Free PMC article.
-
Noninvasive assessment of gene transfer and expression by in vivo functional and morphologic imaging in a rabbit tumor model.PLoS One. 2013 Jun 10;8(6):e62371. doi: 10.1371/journal.pone.0062371. Print 2013. PLoS One. 2013. PMID: 23762226 Free PMC article.
-
Tumor Suppressor Candidate 2 (TUSC2): Discovery, Functions, and Cancer Therapy.Cancers (Basel). 2023 Apr 25;15(9):2455. doi: 10.3390/cancers15092455. Cancers (Basel). 2023. PMID: 37173921 Free PMC article. Review.
-
Mitochondrial Fus1/Tusc2 and cellular Ca2+ homeostasis: tumor suppressor, anti-inflammatory and anti-aging implications.Cancer Gene Ther. 2022 Oct;29(10):1307-1320. doi: 10.1038/s41417-022-00434-9. Epub 2022 Feb 18. Cancer Gene Ther. 2022. PMID: 35181743 Free PMC article. Review.
Cited by
-
Somatostatin receptor type 2 as a radiotheranostic PET reporter gene for oncologic interventions.Theranostics. 2018 May 23;8(12):3380-3391. doi: 10.7150/thno.24017. eCollection 2018. Theranostics. 2018. PMID: 29930736 Free PMC article.
-
Angiotensin II increases gene expression after selective intra-arterial adenovirus delivery in a rabbit model assessed using in vivo SSTR2-based reporter imaging.EJNMMI Res. 2016 Dec;6(1):25. doi: 10.1186/s13550-016-0183-x. Epub 2016 Mar 17. EJNMMI Res. 2016. PMID: 26983635 Free PMC article.
References
-
- American Cancer Society. Cancer Facts & Figures 2011. Atlanta, GA: American Cancer Society; 2011.
-
- Spiro SG, Tanner NT, Silvestri GA, et al. Lung cancer: progress in diagnosis, staging and therapy. Respirology. 2010;15(1):44–50. - PubMed
-
- Scagliotti G. Multimodality approach to early-stage non-small cell lung cancer. Lung Cancer. 2007;57(Suppl 2):S6–S11. - PubMed
-
- Tjuvajev JG, Finn R, Watanabe K, et al. Noninvasive imaging of herpes virus thymidine kinase gene transfer and expression: a potential method for monitoring clinical gene therapy. Cancer Res. 1996;56:4087–4095. - PubMed
-
- Gambhir SS, Barrio JR, Wu L, et al. Imaging of adenoviral-directed herpes simplex virus type 1 thymidine kinase reporter gene expression in mice with radiolabeled ganciclovir. J Nucl Med. 1998;39:2003–2011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
