Androgen receptor increases CD133 expression and progenitor-like population that associate with cisplatin resistance in endometrial cancer cell line
- PMID: 23962788
- PMCID: PMC3936416
- DOI: 10.1177/1933719113497281
Androgen receptor increases CD133 expression and progenitor-like population that associate with cisplatin resistance in endometrial cancer cell line
Abstract
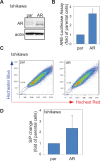
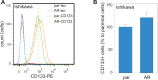
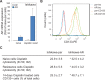
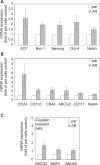
Endometrial cancer (EMC) is a sex steroid hormone-related female malignancy. Androgen and androgen receptor (androgen/AR) signals have been implicated in EMC progression. Cancer stem/progenitor cells (CSPCs) are suspected to link to chemoresistance in patients with EMC. In this study, we examined the androgen/AR roles in cisplatin resistance and CSPC population. We found AR expression increased naive EMC side population, CSPC population, cell migration, and epithelial-mesenchymal transition. Meanwhile, it decreased cisplatin cytotoxic effect on EMC cells. Collaterally, endogenous AR expressions in EMC cells were upregulated in the cisplatin-resisting state. Moreover, AR expression could further enhance CD133 expression, CSPC-related markers, and drug-resistance gene messenger RNA expression in EMC cells. Finally, the AR-associated gene expression might go through indirect regulation. This is the first report revealing AR function on EMC cells' CSPC and cisplatin resistance.
Keywords: AR; cancer stem/progenitor cells; cisplatin resistance; endometrial cancer; side population cell.
Conflict of interest statement
Figures

Similar articles
-
Progestin treatment decreases CD133+ cancer stem cell populations in endometrial cancer.Gynecol Oncol. 2016 Mar;140(3):518-26. doi: 10.1016/j.ygyno.2015.12.022. Epub 2015 Dec 28. Gynecol Oncol. 2016. PMID: 26731726
-
Cisplatin selects for multidrug-resistant CD133+ cells in lung adenocarcinoma by activating Notch signaling.Cancer Res. 2013 Jan 1;73(1):406-16. doi: 10.1158/0008-5472.CAN-12-1733. Epub 2012 Nov 7. Cancer Res. 2013. PMID: 23135908
-
Ligand-independent androgen receptors promote ovarian teratocarcinoma cell growth by stimulating self-renewal of cancer stem/progenitor cells.Stem Cell Res. 2014 Jul;13(1):24-35. doi: 10.1016/j.scr.2014.04.003. Epub 2014 Apr 15. Stem Cell Res. 2014. PMID: 24793306
-
Bladder cancer: Cisplatin response is modulated by AR activity.Nat Rev Urol. 2016 Aug;13(8):437. doi: 10.1038/nrurol.2016.121. Epub 2016 Jun 28. Nat Rev Urol. 2016. PMID: 27349366 Review. No abstract available.
-
Androgen receptor mediated growth control of breast cancer and endometrial cancer modulated by antiandrogen- and androgen-like steroids.J Steroid Biochem Mol Biol. 1996 Jan;56(1-6 Spec No):113-7. doi: 10.1016/0960-0760(95)00228-6. J Steroid Biochem Mol Biol. 1996. PMID: 8603031 Review.
Cited by
-
Androgen/Androgen Receptor Signaling in Ovarian Cancer: Molecular Regulation and Therapeutic Potentials.Int J Mol Sci. 2021 Jul 20;22(14):7748. doi: 10.3390/ijms22147748. Int J Mol Sci. 2021. PMID: 34299364 Free PMC article. Review.
-
Mitochondrial Acetyl-CoA Synthetase 3 is Biosignature of Gastric Cancer Progression.Cancer Med. 2018 Apr;7(4):1240-1252. doi: 10.1002/cam4.1295. Epub 2018 Mar 1. Cancer Med. 2018. PMID: 29493120 Free PMC article.
-
Short androgen receptor poly-glutamine-promoted endometrial cancer is associated with benzo[a]pyrene-mediated aryl hydrocarbon receptor activation.J Cell Mol Med. 2018 Jan;22(1):46-56. doi: 10.1111/jcmm.13291. Epub 2017 Aug 7. J Cell Mol Med. 2018. PMID: 28782227 Free PMC article.
-
Characterization and propagation of tumor initiating cells derived from colorectal liver metastases: trials, tribulations and a cautionary note.PLoS One. 2015 Feb 6;10(2):e0117776. doi: 10.1371/journal.pone.0117776. eCollection 2015. PLoS One. 2015. PMID: 25658706 Free PMC article.
-
Androgen receptor mitigates postoperative disease progression of hepatocellular carcinoma by suppressing CD90+ populations and cell migration and by promoting anoikis in circulating tumor cells.Oncotarget. 2016 Jul 19;7(29):46448-46465. doi: 10.18632/oncotarget.10186. Oncotarget. 2016. PMID: 27340775 Free PMC article.
References
-
- American Cancer Society. Cancer Facts & Figures 2012. Atlanta, GA: American Cancer Society; 2012
-
- Arora V, Quinn MA. Endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2012;26(3):311–324 - PubMed
-
- Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ. Contemporary management of endometrial cancer. Lancet. 2012;379(9823):1352–1360 - PubMed
-
- Pierga JY, Dieras V, Paraiso D, Pouillart P. Chemotherapy of metastatic endometrial carcinoma. Review of the literature. Bull Cancer. 1995;82(12):1005–1018 - PubMed
-
- Vermorken JB, Hoekman K. Chemotherapy for gynecologic malignancies. Curr Opin Oncol. 1995;7(5):457–465 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

