Interspecies interactions stimulate diversification of the Streptomyces coelicolor secreted metabolome
- PMID: 23963177
- PMCID: PMC3747584
- DOI: 10.1128/mBio.00459-13
Interspecies interactions stimulate diversification of the Streptomyces coelicolor secreted metabolome
Abstract
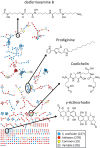
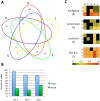
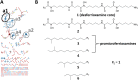
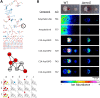
Soils host diverse microbial communities that include filamentous actinobacteria (actinomycetes). These bacteria have been a rich source of useful metabolites, including antimicrobials, antifungals, anticancer agents, siderophores, and immunosuppressants. While humans have long exploited these compounds for therapeutic purposes, the role these natural products may play in mediating interactions between actinomycetes has been difficult to ascertain. As an initial step toward understanding these chemical interactions at a systems level, we employed the emerging techniques of nanospray desorption electrospray ionization (NanoDESI) and matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) imaging mass spectrometry to gain a global chemical view of the model bacterium Streptomyces coelicolor interacting with five other actinomycetes. In each interaction, the majority of secreted compounds associated with S. coelicolor colonies were unique, suggesting an idiosyncratic response from S. coelicolor. Spectral networking revealed a family of unknown compounds produced by S. coelicolor during several interactions. These compounds constitute an extended suite of at least 12 different desferrioxamines with acyl side chains of various lengths; their production was triggered by siderophores made by neighboring strains. Taken together, these results illustrate that chemical interactions between actinomycete bacteria exhibit high complexity and specificity and can drive differential secondary metabolite production.
Importance: Actinomycetes, filamentous actinobacteria from the soil, are the deepest natural source of useful medicinal compounds, including antibiotics, antifungals, and anticancer agents. There is great interest in developing new strategies that increase the diversity of metabolites secreted by actinomycetes in the laboratory. Here we used several metabolomic approaches to examine the chemicals made by these bacteria when grown in pairwise coculture. We found that these interspecies interactions stimulated production of numerous chemical compounds that were not made when they grew alone. Among these compounds were at least 12 different versions of a molecule called desferrioxamine, a siderophore used by the bacteria to gather iron. Many other compounds of unknown identity were also observed, and the pattern of compound production varied greatly among the interaction sets. These findings suggest that chemical interactions between actinomycetes are surprisingly complex and that coculture may be a promising strategy for finding new molecules from actinomycetes.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
