Functional hyperemia and mechanisms of neurovascular coupling in the retinal vasculature
- PMID: 23963372
- PMCID: PMC3824187
- DOI: 10.1038/jcbfm.2013.145
Functional hyperemia and mechanisms of neurovascular coupling in the retinal vasculature
Abstract
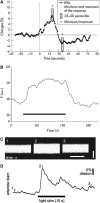
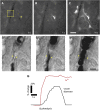
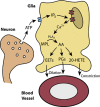
The retinal vasculature supplies cells of the inner and middle layers of the retina with oxygen and nutrients. Photic stimulation dilates retinal arterioles producing blood flow increases, a response termed functional hyperemia. Despite recent advances, the neurovascular coupling mechanisms mediating the functional hyperemia response in the retina remain unclear. In this review, the retinal functional hyperemia response is described, and the cellular mechanisms that may mediate the response are assessed. These neurovascular coupling mechanisms include neuronal stimulation of glial cells, leading to the release of vasoactive arachidonic acid metabolites onto blood vessels, release of potassium from glial cells onto vessels, and production and release of nitric oxide (NO), lactate, and adenosine from neurons and glia. The modulation of neurovascular coupling by oxygen and NO are described, and changes in functional hyperemia that occur with aging and in diabetic retinopathy, glaucoma, and other pathologies, are reviewed. Finally, outstanding questions concerning retinal blood flow in health and disease are discussed.
Figures





References
-
- Braun RD, Linsenmeier RA, Goldstick TK. Oxygen consumption in the inner and outer retina of the cat. Invest Ophthalmol Vis Sci. 1995;36:542–554. - PubMed
-
- Buttery RG, Hinrichsen CF, Weller WL, Haight JR. How thick should a retina be? A comparative study of mammalian species with and without intraretinal vasculature. Vision Res. 1991;31:169–187. - PubMed
-
- Riva CE, Alm A, Pournaras CJ.Ocular circulationIn: Kaufman PL, Alm A, Levin LA, Nilsson SFE, ver Hoeve J, Wu SM, (eds).. Adler's Physiology of the Eye Elsevier: Edinburgh; 2011243–273.
-
- Alm A, Bill A. Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): a study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp Eye Res. 1973;15:15–29. - PubMed
-
- Linsenmeier RA, Padnick-Silver L. Metabolic dependence of photoreceptors on the choroid in the normal and detached retina. Invest Ophthalmol Vis Sci. 2000;41:3117–3123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

