Dna2 is involved in CA strand resection and nascent lagging strand completion at native yeast telomeres
- PMID: 23963457
- PMCID: PMC3795242
- DOI: 10.1074/jbc.M113.472456
Dna2 is involved in CA strand resection and nascent lagging strand completion at native yeast telomeres
Abstract
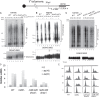
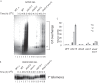
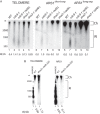
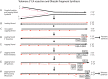
Post-replicational telomere end processing involves both extension by telomerase and resection to produce 3'-GT-overhangs that extend beyond the complementary 5'-CA-rich strand. Resection must be carefully controlled to maintain telomere length. At short de novo telomeres generated artificially by HO endonuclease in the G2 phase, we show that dna2-defective strains are impaired in both telomere elongation and sequential 5'-CA resection. At native telomeres in dna2 mutants, GT-overhangs do clearly elongate during late S phase but are shorter than in wild type, suggesting a role for Dna2 in 5'-CA resection but also indicating significant redundancy with other nucleases. Surprisingly, elimination of Mre11 nuclease or Exo1, which are complementary to Dna2 in resection of internal double strand breaks, does not lead to further shortening of GT-overhangs in dna2 mutants. A second step in end processing involves filling in of the CA-strand to maintain appropriate telomere length. We show that Dna2 is required for normal telomeric CA-strand fill-in. Yeast dna2 mutants, like mutants in DNA ligase 1 (cdc9), accumulate low molecular weight, nascent lagging strand DNA replication intermediates at telomeres. Based on this and other results, we propose that FEN1 is not sufficient and that either Dna2 or Exo1 is required to supplement FEN1 in maturing lagging strands at telomeres. Telomeres may be among the subset of genomic locations where Dna2 helicase/nuclease is essential for the two-nuclease pathway of primer processing on lagging strands.
Keywords: DNA Damage Response; DNA Recombination; DNA Repair; DNA Replication; Dna2 Helicase/Nuclease; Exo1 Nuclease; FEN1; MRX; Okazaki Fragment Processing; Yeast.
Figures



Similar articles
-
The MRX complex plays multiple functions in resection of Yku- and Rif2-protected DNA ends.PLoS One. 2010 Nov 30;5(11):e14142. doi: 10.1371/journal.pone.0014142. PLoS One. 2010. PMID: 21152442 Free PMC article.
-
Multiple pathways regulate 3' overhang generation at S. cerevisiae telomeres.Mol Cell. 2009 Jul 10;35(1):70-81. doi: 10.1016/j.molcel.2009.05.015. Mol Cell. 2009. PMID: 19595717
-
Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends.Cell. 2008 Sep 19;134(6):981-94. doi: 10.1016/j.cell.2008.08.037. Cell. 2008. PMID: 18805091 Free PMC article.
-
Similarities and differences between "uncapped" telomeres and DNA double-strand breaks.Chromosoma. 2012 Apr;121(2):117-30. doi: 10.1007/s00412-011-0357-2. Epub 2011 Dec 28. Chromosoma. 2012. PMID: 22203190 Review.
-
DNA End Resection: Nucleases Team Up with the Right Partners to Initiate Homologous Recombination.J Biol Chem. 2015 Sep 18;290(38):22931-8. doi: 10.1074/jbc.R115.675942. Epub 2015 Jul 31. J Biol Chem. 2015. PMID: 26231213 Free PMC article. Review.
Cited by
-
A Critical Role for Dna2 at Unwound Telomeres.Genetics. 2018 May;209(1):129-141. doi: 10.1534/genetics.118.300809. Epub 2018 Mar 20. Genetics. 2018. PMID: 29559500 Free PMC article.
-
Processing of eukaryotic Okazaki fragments by redundant nucleases can be uncoupled from ongoing DNA replication in vivo.Nucleic Acids Res. 2019 Feb 28;47(4):1814-1822. doi: 10.1093/nar/gky1242. Nucleic Acids Res. 2019. PMID: 30541106 Free PMC article.
-
Genetic Regulation of Dna2 Localization During the DNA Damage Response.G3 (Bethesda). 2015 Jul 10;5(9):1937-44. doi: 10.1534/g3.115.019208. G3 (Bethesda). 2015. PMID: 26163422 Free PMC article.
-
Secondary Mutation-Induced Alternative Splicing Suppresses RNA Splicing Defect of the jhs1 Mutant.Plant Physiol. 2020 Apr;182(4):2025-2034. doi: 10.1104/pp.19.01571. Epub 2020 Feb 13. Plant Physiol. 2020. PMID: 32054782 Free PMC article.
-
To Join or Not to Join: Decision Points Along the Pathway to Double-Strand Break Repair vs. Chromosome End Protection.Front Cell Dev Biol. 2021 Jul 12;9:708763. doi: 10.3389/fcell.2021.708763. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34322492 Free PMC article. Review.
References
-
- Shampay J., Szostak J. W., Blackburn E. H. (1984) DNA sequences of telomeres retained in yeast. Nature 310, 154–157 - PubMed
-
- Walmsley R. W., Chan C. S., Tye B. K., Petes T. D. (1984) Unusual DNA sequences associated with the ends of yeast chromosomes. Nature 310, 157–160 - PubMed
-
- Wellinger R. J., Wolf A. J., Zakian V. A. (1993) Saccharomyces telomeres acquire single-strand TG1–3 tails late in S phase. Cell 72, 51–60 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

