miR-146a-5p circuitry uncouples cell proliferation and migration, but not differentiation, in human mesenchymal stem cells
- PMID: 23963696
- PMCID: PMC3834804
- DOI: 10.1093/nar/gkt666
miR-146a-5p circuitry uncouples cell proliferation and migration, but not differentiation, in human mesenchymal stem cells
Abstract
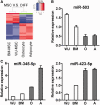
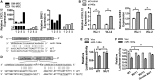
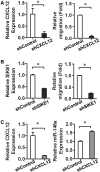
Administration of mesenchymal stem cells (MSCs) has the potential to ameliorate degenerative disorders and to repair damaged tissues. The homing of transplanted MSCs to injured sites is a critical property of engraftment. Our aim was to identify microRNAs involved in controlling MSC proliferation and migration. MSCs can be isolated from bone marrow and umbilical cord Wharton's jelly (BM-MSCs and WJ-MSCs, respectively), and WJ-MSCs show poorer motility yet have a better amplification rate compared with BM-MSCs. Small RNA sequencing revealed that miR-146a-5p is significantly overexpressed and has high abundance in WJ-MSCs. Knockdown of miR-146a-5p in WJ-MSCs inhibited their proliferation yet enhanced their migration, whereas overexpression of miR-146a-5p in BM-MSCs did not influence their osteogenic and adipogenic potentials. Chemokine (C-X-C motif) ligand 12 (CXCL12), together with SIKE1, which is an I-kappa-B kinase epsilon (IKKε) suppressor, is a direct target of miR-146a-5p in MSCs. Knockdown of miR-146a-5p resulted in the down-regulation of nuclear factor kappa-B (NF-κB) activity, which is highly activated in WJ-MSCs and is known to activate miR-146a-5p promoter. miR-146a-5p is also downstream of CXCL12, and a negative feedback loop is therefore formed in MSCs. These findings suggest that miR-146a-5p is critical to the uncoupling of motility and proliferation of MSCs. Our miRNome data also provide a roadmap for further understanding MSC biology.
Figures




References
-
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. - PubMed
-
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat. Med. 1999;5:309–313. - PubMed
-
- Kawada H, Fujita J, Kinjo K, Matsuzaki Y, Tsuma M, Miyatake H, Muguruma Y, Tsuboi K, Itabashi Y, Ikeda Y, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581–3587. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

