Partial proteasome inhibitors induce hair follicle growth by stabilizing β-catenin
- PMID: 23963711
- PMCID: PMC4116182
- DOI: 10.1002/stem.1525
Partial proteasome inhibitors induce hair follicle growth by stabilizing β-catenin
Abstract
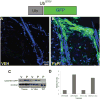
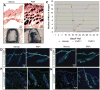
The activation of tissue stem cells from their quiescent state represents the initial step in the complex process of organ regeneration and tissue repair. While the identity and location of tissue stem cells are becoming known, how key regulators control the balance of activation and quiescence remains mysterious. The vertebrate hair is an ideal model system where hair cycling between growth and resting phases is precisely regulated by morphogen signaling pathways, but how these events are coordinated to promote orderly signaling in a spatial and temporal manner remains unclear. Here, we show that hair cycle timing depends on regulated stability of signaling substrates by the ubiquitin-proteasome system. Topical application of partial proteasomal inhibitors (PaPIs) inhibits epidermal and dermal proteasome activity throughout the hair cycle. PaPIs prevent the destruction of the key anagen signal β-catenin, resulting in more rapid hair growth and dramatically shortened telogen. We show that PaPIs induce excess β-catenin, act similarly to the GSK3β antagonist LiCl, and antagonize Dickopf-related protein-mediated inhibition of anagen. PaPIs thus represent a novel class of hair growth agents that act through transiently modifying the balance of stem cell activation and quiescence pathways.
Keywords: Hair growth; Proteasome; Stem cells; Tissue regeneration; Wnt signaling.
Copyright © 2013 AlphaMed Press.
Conflict of interest statement
Figures



References
-
- Keating A. Mesenchymal stromal cells: New directions. Cell Stem Cell. 2012;10:709–716. - PubMed
-
- Anastasia L, Piccoli M, Garatti A, et al. Cell reprogramming: A new chemical approach to stem cell biology and tissue regeneration. Curr Pharm Biotechnol. 2011;12:146–150. - PubMed
-
- Spiegel A, Kalinkovich A, Shivtiel S, et al. Stem cell regulation via dynamic interactions of the nervous and immune systems with the microenvironment. Cell Stem Cell. 2008;3:484–492. - PubMed
-
- Ito M, Liu Y, Yang Z, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. - PubMed
-
- Ito M, Yang Z, Andl T, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

