Desferrioxamine mesylate for managing transfusional iron overload in people with transfusion-dependent thalassaemia
- PMID: 23963793
- PMCID: PMC11491190
- DOI: 10.1002/14651858.CD004450.pub3
Desferrioxamine mesylate for managing transfusional iron overload in people with transfusion-dependent thalassaemia
Abstract
Background: Thalassaemia major is a genetic disease characterised by a reduced ability to produce haemoglobin. Management of the resulting anaemia is through red blood cell transfusions.Repeated transfusions result in an excessive accumulation of iron in the body (iron overload), removal of which is achieved through iron chelation therapy. Desferrioxamine mesylate (desferrioxamine) is one of the most widely used iron chelators. Substantial data have shown the beneficial effects of desferrioxamine, although adherence to desferrioxamine therapy is a challenge. Alternative oral iron chelators, deferiprone and deferasirox, are now commonly used. Important questions exist about whether desferrioxamine, as monotherapy or in combination with an oral iron chelator, is the best treatment for iron chelation therapy.
Objectives: To determine the effectiveness (dose and method of administration) of desferrioxamine in people with transfusion-dependent thalassaemia.To summarise data from trials on the clinical efficacy and safety of desferrioxamine for thalassaemia and to compare these with deferiprone and deferasirox.
Search methods: We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group's Haemoglobinopathies Trials Register. We also searched MEDLINE, EMBASE, CENTRAL (The Cochrane Library), LILACS and other international medical databases, plus ongoing trials registers and the Transfusion Evidence Library (www.transfusionevidencelibrary.com). All searches were updated to 5 March 2013.
Selection criteria: Randomised controlled trials comparing desferrioxamine with placebo, with another iron chelator, or comparing two schedules or doses of desferrioxamine, in people with transfusion-dependent thalassaemia.
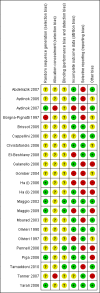
Data collection and analysis: Six authors working independently were involved in trial quality assessment and data extraction. For one trial, investigators supplied additional data upon request.
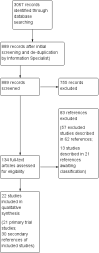
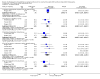
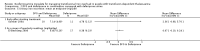
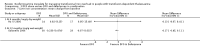
Main results: A total of 22 trials involving 2187 participants (range 11 to 586 people) were included. These trials included eight comparisons between desferrioxamine alone and deferiprone alone; five comparisons between desferrioxamine combined with deferiprone and deferiprone alone; eight comparisons between desferrioxamine alone and desferrioxamine combined with deferiprone; two comparisons of desferrioxamine with deferasirox; and two comparisons of different routes of desferrioxamine administration (bolus versus continuous infusion). Overall, few trials measured the same or long-term outcomes. Seven trials reported cardiac function or liver fibrosis as measures of end organ damage; none of these included a comparison with deferasirox.Five trials reported a total of seven deaths; three in patients who received desferrioxamine alone, two in patients who received desferrioxamine and deferiprone. A further death occurred in a patient who received deferiprone in another who received deferasirox alone. One trial reported five further deaths in patients who withdrew from randomised treatment (deferiprone with or without desferrioxamine) and switched to desferrioxamine alone.One trial planned five years of follow up but was stopped early due to the beneficial effects of a reduction in serum ferritin levels in those receiving combined desferrioxamine and deferiprone treatment compared with deferiprone alone. The results of this and three other trials suggest an advantage of combined therapy with desferrioxamine and deferiprone over monotherapy to reduce iron stores as measured by serum ferritin. There is, however, no evidence for the improved efficacy of combined desferrioxamine and deferiprone therapy against monotherapy from direct or indirect measures of liver iron.Earlier trials measuring the cardiac iron load indirectly by measurement of the magnetic resonance imaging T2* signal had suggested deferiprone may reduce cardiac iron more quickly than desferrioxamine. However, meta-analysis of two trials showed a significantly lower left ventricular ejection fraction in patients who received desferrioxamine alone compared with those who received combination therapy using desferrioxamine with deferiprone.Adverse events were recorded by 18 trials. These occurred with all treatments, but were significantly less likely with desferrioxamine than deferiprone in one trial, relative risk 0.45 (95% confidence interval 0.24 to 0.84) and significantly less likely with desferrioxamine alone than desferrioxamine combined with deferiprone in two other trials, relative risk 0.33 (95% confidence interval 0.13 to 0.84). In particular, four studies reported permanent treatment withdrawal due to adverse events from deferiprone; only one of these reported permanent withdrawals associated with desferrioxamine. Adverse events also occurred at a higher frequency in patients who received deferasirox than desferrioxamine in one trial. Eight trials reported local adverse reactions at the site of desferrioxamine infusion including pain and swelling. Adverse events associated with deferiprone included joint pain, gastrointestinal disturbance, increases in liver enzymes and neutropenia; adverse events associated with deferasirox comprised increases in liver enzymes and renal impairment. Regular monitoring of white cell counts has been recommended for deferiprone and monitoring of liver and renal function for deferasirox.In summary, desferrioxamine and the oral iron chelators deferiprone and deferasirox produce significant reductions in iron stores in transfusion-dependent, iron-overloaded people. There is no evidence from randomised clinical trials to suggest that any one of these has a greater reduction of clinically significant end organ damage, although in two trials, combination therapy with desferrioxamine and deferiprone showed a greater improvement in left ventricular ejection fraction than desferrioxamine used alone.
Authors' conclusions: Desferrioxamine is the recommended first-line therapy for iron overload in people with thalassaemia major and deferiprone or deferasirox are indicated for treating iron overload when desferrioxamine is contraindicated or inadequate. Oral deferasirox has been licensed for use in children aged over six years who receive frequent blood transfusions and in children aged two to five years who receive infrequent blood transfusions. In the absence of randomised controlled trials with long-term follow up, there is no compelling evidence to change this conclusion.Worsening iron deposition in the myocardium in patients receiving desferrioxamine alone would suggest a change of therapy by intensification of desferrioxamine treatment or the use of desferrioxamine and deferiprone combination therapy.Adverse events are increased in patients treated with deferiprone compared with desferrioxamine and in patients treated with combined deferiprone and desferrioxamine compared with desferrioxamine alone. People treated with all chelators must be kept under close medical supervision and treatment with deferiprone or deferasirox requires regular monitoring of neutrophil counts or renal function respectively. There is an urgent need for adequately-powered, high-quality trials comparing the overall clinical efficacy and long-term outcomes of deferiprone, deferasirox and desferrioxamine.
Conflict of interest statement
None known.
Figures




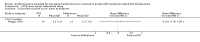



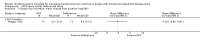
































Update of
-
Desferrioxamine mesylate for managing transfusional iron overload in people with transfusion-dependent thalassaemia.Cochrane Database Syst Rev. 2005 Oct 19;(4):CD004450. doi: 10.1002/14651858.CD004450.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2013 Aug 21;(8):CD004450. doi: 10.1002/14651858.CD004450.pub3. PMID: 16235363 Updated.
References
References to studies included in this review
Abdelrazik 2007 {published data only}
-
- Abdelrazik N. Pattern of iron chelation therapy in Egyptian beta thalassemic patients: Mansoura University Children's Hospital experience. Hematology 2007;12(6):577‐85. - PubMed
-
- Abdelrazik N. Pattern of iron chelation therapy in Egyptian children with beta‐thalassaemia: one‐year Mansoura University Childrens Hospital experience [abstract]. Haematologica 2007;92 Suppl 1:Abstract no: 1146. - PubMed
Aydinok 2005 {published data only}
-
- Aydinok Y, Evans P, Terzi A, Cetiner N, Porter JB. Randomised, prospective evaluation of iron balance, chelation efficiency, urine excretion and NTBI progression with deferiprone (DFP) or deferoxamine (DFO) monotherapy or with combined DFP plus DFO [abstract]. Blood 2005;106(11 Pt 1):2698.
Aydinok 2007 {published data only}
-
- Aydinok Y, El‐Beshlawy A, Orelli‐Leber C, Czarnecki‐Tarabishi C, Manz C. A randomized controlled trial comparing the combination therapy of deferiprone (DFP) and desferrioxamine (DFO) versus DFP or DFO monotherapy in patients with thalassaemia major [abstract]. Blood 2006;108(11):Abstract no: 557.
-
- Aydinok Y, Ulger Z, Nart D, Terzi A, Cetiner N, Ellis G, et al. A randomized, controlled 1‐year study of daily deferiprone plus twice weekly desferrioxamine compared with daily deferiprone monotherapy in patients with thalassaemia major. Haematologica 2007;92(12):1599‐606. - PubMed
-
- Manz C, El‐Beshlawy A, Aydinok Y, Leber C, Czarnecki‐Tarabishi C. A randomized, controlled, prospective clinical study comparing the combination therapy of deferiprone (L1) and desferrioxamine (DFO) with L1 and DFO monotherapy in patients with thalassaemia major [abstract]. Haematologica 2006;91:Abstract no: 515.
Borgna‐Pignatti 1997 {published data only}
-
- Borgna‐Pignatti C, Cohen A. Evaluation of a new method of administration of the iron chelating agent deferoxamine. Journal of Pediatrics 1997;130(1):86‐8. - PubMed
Brissot 2005 {published data only}
-
- Brissot P, Turlin B, Forni GL, Alimena G, Quarta G, Selleslag D, et al. Iron chelation therapy with deferasirox (Exjade, ICL670) or deferoxamine results in reduced hepatocellular inflammation and improved liver function in patients with transfusion‐dependent anemia [abstract]. Blood. 2005; Vol. 106:Abstract no: 823.
Cappellini 2006 {published data only}
-
- Cappellini M, Bejaoui M, Perrotta S, Agaoglu L, Kattamis A, Giardina P, et al. Phase III evaluation of once‐daily, oral therapy with ICL670 (Exjade) versus deferoxamine in patients with beta‐thalassaemia and transfusional hemosiderosis [abstract]. Blood. 2004; Vol. 104:Abstract no: 3619.
-
- Cappellini MD, Bejaoui M, Agaoglu L, Lai ME, Mangiagli A, Strauss G, et al. Patient satisfaction with desferasirox (Exjade ICL670) an oral form of chelation therapy versus deferoxamine an infused chelation therapy [abstract]. Blood 2005;106:Abstract no: 2704.
-
- Cappellini MD, Bejaoui M, Agaoglu L, Porter J, Coates T, Jeng M. Prospective evaluation of patient‐reported outcomes during treatment with deferasirox or deferoxamine for iron overload in patients with beta‐thalassemia. Clinical Therapeutics 2007;29(5):909‐17. - PubMed
-
- Cappellini MD, Cohen A, Piga A, Bejaoui M, Perrotta S, Agaoglu L, et al. A phase 3 study of deferasirox (ICL670), a once daily oral iron chelator in patients with beta‐thalassaemia. Blood 2006;107(9):3455‐62. - PubMed
-
- Cohen AR, Glimm E, Porter KJB. Effect of transfusional iron intake on response to chelation therapy in beta‐thalassemia‐major. Blood 2008;111(2):583‐7. - PubMed
Christoforidis 2006 {published data only}
-
- Christoforidis A, Tsatra I, Zevgaridou E, Koussi A, Tsitourides I, Athanassiou‐Metaxa M. Evaluation of myocardial deposition assessed with MRI in young thalassaemia patients receiving one year of deferasirox versus deferoxamine [abstract]. Haematologica 2006;91:Abstract no: 209.
El‐Beshlawy 2008 {published data only}
-
- El‐Beshlawy A, Manz C, Naja M, Eltagui M, Tarabishi C, Youssry I, et al. Iron chelation in thalassaemia: combined or monotherapy? The Egyptian experience. Annals of Hematology 2008;87(7):545‐50. - PubMed
Galanello 2006 {published data only}
-
- Galanello R, Kattamis A, Piga A, Fischer R, Leoni G, Ladis V, et al. A prospective randomised controlled trial on the safety and efficacy of alternating deferoxamine and deferiprone in the treatment of iron overload in patients with thalassaemia. Haematologica 2006;91(9):1241‐3. - PubMed
-
- Galanello R, Kattamis A, Piga A, Tricta F. Safety and efficacy of alternate desferrioxamine and deferiprone compared to desferrioxamine alone in the treatment of iron overload in transfusion‐dependent thalassemia patients [abstract]. Blood 2004;104(11 Pt 1):Abstract no: 3611.
Gomber 2004 {published data only}
-
- Gomber S, Saxena R, Madan N. Comparative efficacy of desferrioxamine, deferiprone and in combination on iron chelation in thalassaemic children. Indian Paediatrics 2004;41(1):21‐7. - PubMed
Ha (i) 2006 {published data only}
-
- Ha SY, Chik KW, Ling SC, Lee AC, Luk CW, Lam CW, et al. A randomised controlled study evaluating the safety and efficacy of deferiprone treatment in thalassaemia major patients from Hong Kong. Hemoglobin 2006;30(2):263‐74. - PubMed
-
- Ha SY, Chik KW, Ling SC, Lee ACW. A randomised controlled study on safety and efficacy of iron chelation therapy with (L1) deferiprone in thalassaemic major patients [abstract]. Conference proceedings for the 13th International Conference on Oral Chelation in the treatment of thalassaemia and other diseases. 2003:Abstract no: 72.
Ha (ii) 2006 {published data only}
-
- Ha SY, Chik KW, Ling SC, Lee AC, Luk CW, Lam CW, et al. A randomised controlled study evaluating the safety and efficacy of deferiprone treatment in thalassaemia major patients from Hong Kong. Hemoglobin 2006;30(2):263‐74. - PubMed
-
- Ha SY, Chik KW, Ling SC, Lee ACW. A randomised controlled study on safety and efficacy of iron chelation therapy with (L1) deferiprone in thalassaemic major patients [abstract]. Conference Proceedings for the 13th International Conference on Oral Chelation in the Treatment of Thalassaemia and Other Diseases. 2003:Abstract no: 72.
Maggio 2002 {published data only}
-
- Galia M, Midiri M, Bartolotta V, Morabito A, Rizzo M, Mangiagli A, et al. Potential myocardial iron content evaluation by magnetic resonance imaging in thalassemia major patients treated with deferoxamine or deferiprone during a randomized multicenter prospective clinical study. Hemoglobin 2003;27(2):63‐76. - PubMed
-
- Maggio A, Capra M, Ciaccio C, Magnano C, Rizzo M, Mangiagli A, et al. Evaluation of efficacy of L1 versus desferrioxamine by clinical randomized multicentric study [abstract]. Blood 1999;94(10 Suppl 1 Pt 2):34b.
-
- Maggio A, D'Amico G, Morabito A, Capra M, Ciaccio C, Cianciulli P, et al. Deferiprone versus deferoxamine in patients with thalassemia major: a randomized clinical trial. Blood Cells, Molecules and Diseases 2002;28(2):196‐208. - PubMed
Maggio 2009 {published data only}
-
- Maggio A, Vitrano A, Capra M, Cuccia L, Gagliardotto F, Filosa A, et al. Decrease of mortality during deferiprone treatments: results from a large randomised cohort of thalassemia major patients under the auspices of the Italian Society for Thalassemia and Hemoglobinopathies [abstract]. Blood 2008;112:Abstract no: 3885.
-
- Maggio A, Vitrano A, Capra M, Cuccia L, Gagliardotto F, Filosa A, et al. Improving survival with deferiprone treatment in patients with thalassemia major: a prospective multicenter randomised clinical trial under the auspices of the Italian Society for Thalassemia and Hemoglobinopathies. Blood Cells, Molecules and Diseases 2009;42(3):247‐51. - PubMed
-
- Maggio A, Vitrano A, Capra M, Cuccia L, Gagliardotto F, Filosa A, et al. Long‐term sequential deferiprone‐deferoxamine versus deferiprone alone for thalassaemia major patients: a randomized clinical trial. British Journal of Haematology 2009;145(2):245‐54. - PubMed
-
- Pantalone GR, Maggio A, Vitrano A, Capra M, Cuccia L, Gagliardotto F, et al. Sequential alternating deferiprone and deferoxamine treatment compared to deferiprone monotherapy: main findings and clinical follow‐up of a large multicenter randomized clinical trial in beta‐thalassemia major patients. Hemoglobin 2011;35(3):206‐16. - PubMed
Mourad 2003 {published data only}
-
- Mourad FH, Hoffbrand AV, Sheikah‐Taha M, Koussa S, Khoriaty AI, Taher A. Comparison between desferrioxamine and combined therapy with desferrioxamine and deferiprone in iron overloaded thalassaemia patients. British Journal of Haematology 2003;121(1):187‐9. - PubMed
Olivieri 1990 {published data only}
-
- Olivieri NF, Koren G, Hermann C, Bentur Y, Chung D, Klein J, et al. Comparison of oral iron chelator L1 and desferrioxamine in iron‐loaded patients. Lancet 1990;336(8726):1275‐9. - PubMed
-
- Olivieri NF, Koren G, Louis P, Freedman M, McClelland R, Templeton D. Studies of the oral chelator 1,2‐dimethyl‐3‐hydroxypyrid‐4‐one in thalassemia patients. Seminars in Hematology 1990;27(2):101‐4. - PubMed
-
- Olivieri NF, Templeton DM, Koren G, Chung D, Hermann C, Freedman MH, et al. Evaluation of the oral iron chelator 1,2‐dimethyl‐3‐hydroxypyrid‐4‐one (L1) in iron‐loaded patients. Annals of the New York Academy of Sciences 1990;612:369‐77. - PubMed
Olivieri 1997 {published data only}
-
- ApoPharma Inc. Oncologic drugs advisory committee briefing document: NDA #21‐825. US Food and Drug Administration 2011; Vol. Appendix A:100‐101 (http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/O...).
-
- Olivieri NF, Brittenham GM. Evidence of progression of myocardial iron loading as determined by magnetic resonance imaging (MRI) in thalassemia patients during treatment with deferiprone (L1) and deferoxamine (DFO) [abstract]. Blood 1999;94(10 Suppl 1 Pt 2):35b.
-
- Olivieri NF, Brittenham GM. Final results of the randomized trial of deferiprone (L1) and deferoxamine (DFO) [abstract]. Blood 1997;90:264a.
-
- Olivieri NF, Brittenham GM, Armstrong SAM, Basran RK, Daneman R, Daneman N, et al. First prospective randomized trial of the iron chelators deferiprone (L1) and deferoxamine [abstract]. Blood 1995;86(10 Suppl 1):249a.
Pennell 2006 {published data only}
-
- Pennell DJ, Berdoukas V, Karagiorga M, Ladis V, Piga A, Aessops A, et al. Randomized controlled trial of deferiprone or deferoxamine in beta‐thalassemia major patients with asymptomatic myocardial siderosis. Blood 2006;107(9):3738‐44. - PubMed
Piga 2006 {published data only}
-
- Cappellini MD, Galanello R, Piga A, Forni GL, Opitz H, Ford JM, et al. Pharmacokinetics (PK) profile of the new oral iron chelator ICL670 after 6 months of treatment in a Phase II study in patients with transfusional hemosiderosis [abstract]. Proceedings of the 8th Congress of the European Haematology Association. 2003:Abstract no: 0909.
-
- Cappellini MD, Galanello R, Piga A, Forni GL, Zanaboni L, Muroni P, et al. Update on the effects of ICL670, a novel tridentate oral iron chelator on liver iron concentration in patients with transfusion dependent iron overload [abstract]. Haematology Journal. 2002; Vol. Suppl 1:Abstract no: 0610.
-
- Piga A, Galanello R, Cappelli MD, Forni GL, Lupo G, Ford JM, et al. Phase II study of ICL670, an oral iron chelator in adult thalassaemia patients with transfusional iron overload: efficacy, safety, pharmacokinetics (PK) and pharmacodynamics (PD) after 18 months of therapy [abstract]. Blood. 2003; Vol. 102:Abstract no: 412.
-
- Piga A, Galanello R, Cappellini MD, Forni GL, Opitz H, Ford JM, et al. Phase II study of oral chelator ICL670 in thalassamia patients with transfusional iron overload: efficacy, safety, pharmacokinetics (PK) and pharmacodynamics (PD) after 6 months of therapy [abstract]. Blood 2002;100:Abstract no: 5a.
-
- Piga A, Galanello R, Forni GL, Cappellini MD, Origa R, Zappu A, et al. Randomised phase II trial of deferasirox (Exjade ICL670), a once daily, orally administered iron chelator, in comparison to deferoxamine in thalassaemia patients with transfusional iron overload. Haematologica 2006;91(7):873‐80. - PubMed
Tamaddoni 2010 {published data only}
-
- Tamaddoni A, Ramezani MS. Comparison between deferoxamine and combined therapy with deferoxamine and deferiprone in iron overloaded thalassemia patients. Iranian Red Crescent Medical Journal 2010;12(6):655‐9.
Tanner 2007 {published data only}
-
- Alpendurada F, Carpenter JP, Smith GC, Tanner MA, Banya W, Galanello R, et al. Effect of myocardial iron removal on right ventricular function: insights from a randomized, placebo controlled, double blind trial in thalassemia major [abstract]. European Heart Journal; Proceedings of the European Society of Cardiology, ESC Congress, Stockholm, Sweden. 2010.
-
- Porter JB, Tanner MA, Pennell DJ, Eleftheriou P. Improved myocardial T2* in transfusion dependent anemias receiving ICL670 (Deferasirox) [abstract]. Blood 2005;106:Abstract no: 3600.
-
- Tanner M. The effect of combined therapy with deferoxamine and deferiprone on myocardial iron and endothelial function in thalassaemia major: a randomised controlled trial using cardiovascular magnetic resonance [abstract]. Proceedings of the European Haematology Association 11th Congress. 2006:Abstract no: 0517.
-
- Tanner MA, Galanello R, Dessi C, Agus A, Smith GC, Westwood MA, et al. Improved endothelial function with combined chelation therapy in thalassaemia major [abstract]. Blood 2006;108:Abstract no: 1770.
-
- Tanner MA, Galanello R, Dessi C, Smith GC, Westwood MA, Agus A, et al. A randomized, placebo‐controlled, double‐blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassaemia major using cardiovascular magnetic resonance. Circulation 2007;115(14):1876‐84. - PubMed
Yarali 2006 {published data only}
-
- Yarali N, Fisgin T, Duru F, Lara A, Ecin N, Fitoz S, et al. Subcutaneous bolus injection of deferoxamine is an alternative method to subcutaneous continuous infusion. Journal of Pediatric Hematology and Oncology 2006;28(1):11‐6. - PubMed
References to studies excluded from this review
Agouzal 2010 {published data only}
Aldouri 1990 {published data only}
-
- Aldouri MA, Wonke B, Hoffbrand AV, Flynn DM, Ward SE, Agnew JE, et al. High incidence of cardiomyopathy in beta‐thalassaemia patients receiving regular transfusion and iron chelation: reversal by intensified chelation. Acta Haematologica 1990;84(3):113‐7. - PubMed
al Refaie 1992 {published data only}
-
- Al‐Refaie FN, Wickens DG, Wonke B, Kontoghiorghes GJ, Hoffbrand AV. Serum non‐transferrin‐bound iron in beta‐thalassaemia major patients treated with desferrioxamine and L1. British Journal of Haematology 1992;82:431‐6. - PubMed
Anderson 2002 {published data only}
-
- Anderson LJ, Wonke B, Prescott E, Holden S, Walker JM, Pennell DJ. Comparison of effects of oral deferiprone and subcutaneous desferrioxamine on myocardial iron concentrations and ventricular function in beta‐thalassaemia. Lancet 2002;360(9332):516‐20. - PubMed
Andres 1980 {published data only}
-
- Andres VJ, Glatzel E, Ihle R. On the treatment of iron overload with the iron chelating agent Desferrioxamine (Desferal) [Zur Behandlung der Eisenuberladhung mit dem Eisenchelator Desferrioxamin (Desferal)]. Dt. Gesundh‐Wesen 1980;35(7):273‐7.
Athanassiou‐Metaxa 2004 {published data only}
-
- Athanassiou‐Metaxa M, Kousi A, Hatzipantelis E, Tsatra I, Ikonomou M, Perifanis V, et al. Combined chelation therapy with deferiprone and desferrioxamine in iron overloaded ß‐thalassemia patients. Haematologica 2004; Vol. 89, issue 3:ELT07. - PubMed
Barry 1974 {published data only}
Bartfay 1999 {published data only}
-
- Bartfay WJ, Lehotay DC, Sher GD, Bartfay E, Tyler B, Luo X, et al. Erythropoiesis: comparison of cytotoxic aldehyde generation in beta‐thalassaemia patients chelated with deferoxamine or deferiprone (L1) versus no chelation. Haematology 1999;4(1):67‐76. - PubMed
Borgna‐Pignatti 1989 {published data only}
-
- Borgna‐Pignatti C, Zurlo MG, DeStefano P, DiGregorio F, DiPalma A, Piga A, et al. Survival in thalassaemia with conventional treatment. Progress in Clinical and Biological Research 1989;309:27‐33. - PubMed
Brittenham 2003b {published data only}
-
- Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassaemia major. New England Journal of Medicine 1994;331(9):567‐73. - PubMed
B‐Weintrob 1990 {published data only}
-
- Bronspiegel‐Weintrob N, Olivieri NF, Tyler B, Andrews DF, Freedman M, Holland J. Effect of age at the start of iron chelation therapy on gonadal function in beta‐thalassaemia major. New England Journal of Medicine 1990;323(11):713‐9. - PubMed
Calleja 1998 {published data only}
-
- Calleja EM, Shen JY, Lesser M, Grady RW, New MI, Giardina PJ. Survival and morbidity in transfusion‐dependent thalassaemic patients on subcutaneous desferrioxamine chelation. Nearly two decades of experience. Annals New York Academy of Science 1998;850:469‐70. - PubMed
Cassinerio 2012 {published data only}
-
- Cassinerio E, Roghi A, Pedrotti P, Brevi F, Zanaboni L, Graziadei G, et al. Cardiac iron removal and functional cardiac improvement by different iron chelation regimens in thalassemia major patients. Annals of Hematology 2012;91(9):1443‐9. - PubMed
Christoforidis 2007 {published data only}
-
- Christioforidis A, Zevgaridou E, Tsatra I, Perifanis V, Vlachaki E, Papassoitirou I, et al. Urinary iron excretion in young thalassemic patients receiving combined chelation treatment with deferoxamine and deferiprone. Journal of Paediatric Haematology and Oncology 2007;29(9):598‐601. - PubMed
Cianciulli 1993 {published data only}
-
- Cianciulli P, Forte L, Sorrentino F, Palombi M, Papa G, Marciani MG. Intensive long‐term intravenous iron‐chelation therapy with deferoxamine in thalassaemic patients. Bone Marrow Transplantation 1993;12 Suppl 1:5‐8. - PubMed
Cianciulli 1994 {published data only}
-
- Cianciulli P, Sollecito D, Sorrentino F, Forte L, Gilradi E, Massa A. Early detection of nephrotoxic effects in thalassemic patients receiving desferrioxamine therapy. Kidney International 1994;46(2):467‐70. - PubMed
Davies 1983 {published data only}
-
- Davies SC, Marcus RE, Hungerford JL, Miller MH, Arden GB, Huehns ER. Ocular toxicity of high‐dose intravenous desferrioxamine. Lancet 1983;2(8343):181‐4. - PubMed
De Sanctis 1994 {published data only}
-
- Sanctis V, Katz M, Vullo C, Bagni B, Ughi M, Wonke B. Effect of different treatment regimes on linear growth and final height in beta‐thalassaemia major. Clinical Endocrinology 1994;40(6):791‐8. - PubMed
DeVirgiliis 1988 {published data only}
-
- DeVirgiliis S, Congia M, Frau F, Argiolu F, Diana G, Cucca F, et al. Deferoxamine‐induced growth retardation in patients with thalassaemia major. Journal of Pediatrics 1988;113(4):661‐9. - PubMed
Drakonaki 2010 {published data only}
-
- Drakonaki EE, Maris TG, Maragaki S, Klironomos V, Papadakis A, Karantanas AH. Deferoxamine versus combined therapy for chelating liver, spleen and bone marrow iron in beta‐thalassemic patients: a quantitative magnetic resonance imaging study. Hemoglobin 2010;34(1):95‐106. - PubMed
Elalfy 2006 {published data only}
-
- Elalfy MS, Abdin I. Liver status in a cohort of polytransfused b‐thalasseamie major (BTM) on long term desferrioxamine (DFO) or Deferiprone (L1) [abstract]. Blood 2006;108:Abstract no: 3732.
Eleftheriou 2006 {published data only}
-
- Eleftherioiu P, Tanner M, Pennell D, Porter J. Response of myocardial T2* to oral deferasirox monotherapy for 1 year in 29 patients with transfusion‐dependent anaemias: a subgroup analysis [abstract]. Haematologica 2006;91 Suppl 1:Abstract no: 366.
-
- Porter KJB, Tanner MA, Pennell DJ, Eleftheriou P. Improved myocardial mT2* in transfusion dependent anemias receiving ICL670 (Deferasirox) [abstract]. Blood 2005;106:Abstract no: 3600.
Fragatou 2007 {published data only}
-
- Fragatou S, Politis C, Vandiadi K, Tsiapras D, Douskou M. Cardiac function in thalassaemics on combined deferoxamine and deferiprone therapy [abstract]. Haematologica 2007;92:299, Abstract no: 0801.
Galanello 1999 {published data only}
-
- Galanello R, Kattamis C, Athanassiou M, Quarta G, Ballati G, Zoumbos N, et al. A depot formulation of desferrioxamine (ICL749B): update on the dose‐finding program [abstract]. Blood 1999;94:32b.
Gaziev 1995 {published data only}
-
- Gaziev D, Giardini C, Angelucci E, Polchi P, Galimberti M, Baronciani D, et al. Intravenous chelation therapy during transplantation for thalassaemia. Haematologica 1995;80(4):300‐4. - PubMed
Gharagozloo 2009 {published data only}
-
- Gharagozloo M, Moayedi B, Zakerinia M, Hamidi M, Karimi M, Maracy M, et al. Combined therapy of silymarin and desferrioxamine in patients with beta‐thalassemia major: a randomized double‐blind clinical trial. Fundamental and Clinical Pharmacology 2009;23(3):359‐65. - PubMed
Goulas 2012 {published data only}
-
- Goulas V, Kourakli‐Symeonidis A, Camoutisis C. Comparative effects of three iron chelation therapies on the quality of life in Greek patients with homozygous transfusion‐dependent beta‐thalassemia. http://www.hindawi.com/isrn/hematology/2012/139862/cta/ (accessed 01 August 2013). - PMC - PubMed
Grady 2001 {published data only}
-
- Grady RW, Berdoukas V, Rachmilewitz EA, Galanello R, Borgna‐Pignatti C, Ladis V. When deferiprone and desferrioxamine are combined iron excretion is enhanced [abstract]. Blood 2001;98(11 Pt 1):494a.
Graziano 1978 {published data only}
-
- Graziano JH, Markenson A, Miller DR, Chang H, Bestak M, Meyers P, et al. Chelation therapy in beta‐thalassaemia major. I. Intravenous and subcutaneous deferoxamine. Journal of Pediatrics 1978;92(4):648‐52. - PubMed
Hussain 1976 {published data only}
-
- Hussain MAM, Flynn DM, Green N, Hussein S, Hoffbrand AV. Subcutaneous infusion and intramuscular injection of desferrioxamine in patients with transfusional iron overload. Lancet 1976;2(7998):1278‐80. - PubMed
Kattamis 1981 {published data only}
-
- Kattamis C, Fitsialos J, Sinopoulou C. Oral desferrioxamine in young patients with thalassaemia. Lancet 1981;1(8210):51. - PubMed
Kattamis 2003 {published data only}
-
- Kattamis A, Kassou C, Berdousi H, Ladis V, Papassotiriou I, Kattamis C. Combined therapy with desferrioxamine and deferiprone in thalassemic patients: effect on urinary iron excretion. Haematologica 2003;88(12):1423‐5. - PubMed
Keshtkaran 2013 {published data only}
Kontoghiorghes 1987 {published data only}
Lai 2010 {published data only}
-
- Lai ME, Grady RW, Vacquer S, Pepe A, Carta MP, Bina P, et al. Increased survival and reversion of iron‐induced cardiac disease in patients with thalassemia major receiving intensive combined chelation therapy as compared to deferoxamine alone. Blood Cells, Molecules and Diseases 2010;45(2):136‐9. - PubMed
Li 2000 {published data only}
-
- Li CK, Lai DH, Shing MMK, Chik KW, Lee V, Yuen PMP. Early iron reduction programme for thalassaemia patients after bone marrow transplantation. Bone Marrow Transplantation 2000;25(6):653‐6. - PubMed
Loebstein 1997 {published data only}
-
- Loebstein R, Dalal I, Nisbet‐Brown E, Berkovitch M, Meydan N, Andrews D, et al. Immune function in patients with beta‐thalassamia receiving the orally active iron‐chelating agent deferiprone. British Journal of Haematology 1997;98(3):597‐600. - PubMed
Nienhuis 1976 {published data only}
-
- Nienhuis AW, Delea C, Aamodt R, Bartter F, Anderson WF. Evaluation of desferrioxamine and ascorbic acid for the treatment of chronic iron overload. Birth Defects: Original Article Series 1976;12(8):177‐85. - PubMed
Olivieri 1992 {published data only}
-
- Olivieri NF, Berriman AM, Tyler BJ, Davis SA, Francombe WH, Liu PP. Reduction in tissue iron stores with a new regimen of continuous ambulatory intravenous deferoxamine. American Journal of Haematology 1992;41(1):61‐3. - PubMed
Peng 2006 {published data only}
-
- Peng CT, Wu KH, Tsai CC, Ysai CH. Deferirpone in patients with beta‐thalassamiea major for 4 years in the Chinese population in Taiwan [abstract]. European Journal of Clinical Investigation 2004;34 Suppl 1:20‐61, Abstract no:138.
-
- Peng CT, Wu KH, Tsai CH, Yang CP, Wang LW, Jang RC, et al. Study of deferiprone or deferoxamine versus combination therapy in iron‐loaded thalassaemia patients in Taiwan [abstract]. Blood 2006;18:Abstract no: 3736.
Pennell 2012 {published data only}
-
- Pennell D, Porter JB, Cappellini MD, Chan L, El‐Beshlawi A, Aydinok Y, et al. Continued improvement and normalization of myocardial T2* in patients with beta‐thalassemia major treated with deferasirox (Exjade) for up to 3 years. Blood. 2010; Vol. 116 (21).
Pepe 2006 {published data only}
-
- Pepe A, Lombardi M, Positano V, Cracolici E, Capra M, Malizia R, et al. Evaluation of the efficacy of oral deferiprone in beta‐thalassaemia major by multislice multiecho T2*. European Journal of Haematology 2006;76(3):183‐92. - PubMed
Piga 2007 {published data only}
-
- Piga A, Vichinsky E, Forni GL, Killinc Y, Maseruka H, Kattamis A. Long‐term efficacy and safety with Deferasirox (Exjade ICL670) a once‐daily oral iron chelator in pediatric patients [abstract]. Blood 2007;110:Abstract no: 2774.
Pippard 1978b {published data only}
-
- Pippard MJ, Callender ST, Weatherall DJ. Intensive iron‐chelation therapy with desferrioxamine in iron‐loading anaemias. Clinical Science and Molecular Medicine 1978;54(1):99‐106. - PubMed
Propper 1977 {published data only}
-
- Propper RD, Cooper B, Rufo R, Nienhuis AW, Anderson WF, Bunn HF. Continuous subcutaneous administration of deferoxamine in patients with iron overload. New England Journal of Medicine 1977;297(8):418‐23. - PubMed
Ricchi 2010 {published data only}
-
- Ricchi P, Ammirabile M, Spasiano A, Costantini S, Cinque P, Matola T, et al. Combined chelation therapy in thalassemia major with deferiprone and desferrioxamine: a retrospective study. European Journal of Haematology 2010;85(1):36‐42. - PubMed
Russo 1990 {published data only}
-
- Russo G. Iron chelating therapy in thalassaemia: current problems. Haematologica 1990;75 Suppl 5:848‐8. - PubMed
Taher 2001 {published data only}
-
- Taher A, Sheikh‐Taha M, Koussa S, Inati A, Neeman R, Mourad F. Comparison between deferoxamine and deferiprone (L1) in iron‐loaded patients. European Journal of Haematology 2001;67(1):30‐4. - PubMed
Tanner 2008 {published data only}
Torcharus 1993 {published data only}
-
- Torcharus K, Withayathawornwong W, Sriphaisal T, Krutvacho T, Arnutti P, Suwanasophorn C. High transfusion in children with beta‐thalassaemia/Hb E: clinical and laboratory assessment of 18 cases. Southeast Asian Journal of Tropical Medicine and Public Health 1993;24 Suppl 1:96‐9. - PubMed
Tsakok 2004 {published data only}
-
- Tsakok AD. Deferiprone versus deferoxamine in patients with thalassaemia major: a randomized clinical trial. Blood Cells, Molecules and Diseases 2004;32:139‐40. - PubMed
Vannasaeng 1991 {published data only}
-
- Vannasaeng S, Fucharoen S, Pootrakul P, Ploybutr S, Yansukon P. Pituitary function in thalassemic patients and the effect of chelation therapy. Acta Endocrinologica (Copenh) 1991;124(1):23‐30. - PubMed
Vlachaki 2007 {published data only}
-
- Vlachaki E, Ioannidou‐Papagiannaki E, Haralambidou‐Vranitsa SH, Perifanis V, Tsigga A, Klonizakis I, et al. Progenitor haemopoietic cells in the peripheral blood of thalassemic patients with desferrioxamine or deferiprone chelation therapy [abstract]. Hematology 2005;90 Suppl 2:Abstract no: 1065.
-
- Vlachaki E, Ioannidou‐Papagiannaki E, Tziomalos K, Haralambidou‐Vranitsa S, Perifanis V, Klonizakis I, et al. Peripheral blood haematopoietic progenitor cells in patients with beta thalassaemia major receiving desferrioxamine or deferiprone as chelation therapy. European Journal of Haematology 2007;78(1):48‐51. - PubMed
Walter 2008 {published data only}
-
- Walter PB, Macklin EA, Porter J, Evans P, Kwiatkowski JL, Neufeld EJ, et al. Inflamation and oxidant stress in beta‐thalassaemia patients treated with iron chelators deferasirox (ICL670) or deferoxamine: an ancillary study of Novartis CICL670A0107. Haematologica 2008;93(6):817‐25. - PubMed
Wang 2006 {published data only}
-
- Wang CH, Wu KH, T FJ, Peng CT, T CH. Comparison of oral and subcutaneous iron chelation therapies in the prevention of major endocrinopathies in beta‐thalassaemia major patients. Hemoglobin 2006;30(2):257‐62. - PubMed
Wonke 1998 {published data only}
-
- Wonke B, Wright C, Hoffbrand AV. Combined therapy with deferiprone and desferrioxamine. British Journal of Haematology 1998;103(2):361‐4. - PubMed
Zareifar 2009 {published data only}
-
- Zareifar S, Jabbari A, Cohan N, Haghpanah S. Efficacy of combined desferrioxamine and deferiprone versus single desferrioxamine therapy in patients with major thalassemia. Archives of Iranian Medicine 2009;12(5):488‐91. - PubMed
References to studies awaiting assessment
Alpendurada 2012 {published data only}
Aydinok 2012 {published data only}
-
- Aydinok Y, Evans P, Manz CY, Porter JB. Probing the origin of chelatable iron during deferiprone and combination therapies: insights from plasma NTBI and LPI determinations [abstract]. Blood 2010;116(21):Abstract no: 5158.
Badawi 2010 {published data only}
-
- Badawy S, Hassan TH, Hesham MAA, Badr MA. Evaluation of iron chelation therapy in beta‐thalassemic patients in Zagazig University Hospital. Pediatric Blood and Cancer; Proceedings of the 23rd Annual Meeting of the American Society of Pediatric Hematology/Oncology, ASPHO, Montreal, Canada. 2010:799‐800, Poster no: 124.
-
- Hassan T, Badr M, Hesham M, Badawy S. Evaluation of iron chelation therapy in beta‐thalassemia major patients in East Delta of Egypt. Haematologica 2010;95 Suppl 2:701, Abstract no: 1810.
Canatan 1999 {published data only}
-
- Canatan D, Temimhan N, Dincer N, Ozsancak A, Oguz N, Temimhan M. Continuous desferrioxamine infusion by an infusor in thalassaemia major. Acta Paediatrica 1999;88(5):550‐2. - PubMed
Evans 2011 {published data only}
-
- Evans P, Aydinok Y, Manz C, Porter J. Origin of chelatable iron during deferiprone and combination therapies: Insights from plasma NTBI and LPI. American Journal of Hematology 2011;86:9: E84.
Jain 2011 {published data only}
-
- Jain R, Perkins J, Johnson S, Harimoorthy V, Desai P, Chudgar U, et al. A prospective study for determination of the mean red cell transfusion requirement compared on the basis of iron overload and type of chelation therapy in multiply transfused thalassaemia major patients. Transfusion Medicine. 29th Annual Scientific Meeting of the British Blood Tranfusion Society, Glasgow, UK 2011;21:46‐7.
Kompany 2009 {published data only}
-
- Kompany F, Mohammadi S, Sigari N, Hadizadeh N, Rezaie N, Gharibi FSM. Comparative efficacy of deferrioxamine and combination of deferiprone and deferrioxamine on echocardiographic indices in beta thalassemic patients. Scientific Journal of Kurdistan University of Medical Sciences 2009;14(2):21‐30.
Maggio 2012 {published data only}
-
- Maggio A, Capra M, Cuccia L, Gagliardotto F, Rigano P, Calvaruso G, et al. Long‐term use of deferiprone enhances significantly the left ventricular ejection function in thalassemia major [abstract]. ASH Annual Meeting Abstracts; 53rd American Society of Hematology (ASH) Annual Meeting, 10‐13 December 2011, San Diego, USA. 2011; Vol. 118:21; Abstract no: 5302.
-
- Maggio A, Vitrano A, Lucania G, Capra M, Cuccia L, Gagliardotto F, et al. Long‐term use of deferiprone significantly enhances left‐ventricular ejection function in thalssemia major patients. American Journal of Hematology 2012;87(7):732‐3. - PubMed
Mirbehbahani 2012 {published data only}
-
- Mirbehbahani N, Jahazi A, Rahim AHH. The effect of combined therapy with deferoxamine and deferiprone on serum ferritin level of beta‐thalassemic patients. Hematology 2012;17(3):183‐6. - PubMed
N0277104959 {published data only}
-
- N0277104959. A randomised controlled prospective trial using Ferriprox versus placebo and conventional intravenous and subcutaneous iron chelation. http://www.nihr.ac.uk/Profile/Pages/NRRResults.aspx?publication_id=N0277... (accessed 1 September 2009).
NCT00004982 {published data only}
-
- NCT00004982. Combination Iron Chelation Therapy. http://www.clinicaltrials.gov/ct2/show/NCT00004982?term=NCT00004982&... (accessed 5 September 2011).
NCT00115349 {published data only}
-
- NCT00115349. Combination therapy compared with single‐drug therapy in patients with cardiac diseases. http://www.clinicaltrials.gov/ct2/show/NCT00115349?term=NCT00115349&... (accessed 5 September 2011).
Pantalone 2011 {published data only}
-
- Pantalone GR, Maggio A, Vitrano A, Capra M, Cuccia L, Gagliardotto F, et al. Sequential alternating deferiprone and deferoxamine treatment compared to deferiprone monotherapy: Main findings and clinical follow‐up of a large multicenter randomized clinical trial in beta‐thalassemia major patients. Hemoglobin 2011;35(3):206‐16. - PubMed
Pennell 2010 {published data only}
-
- Pennell D, Porter J, Piga A, El‐Alfy M, El‐Beshlawi A, Kilinc Y, et al. Prevalence of cardiac iron overload in patients with transfusion‐dependent anemias: data from the ranodomized, active‐controlled deferasirox CORDELIA trial. Haematologica. 17th Congress of the European Hematology Assocation, Amsterdam, The Netherlands, 14‐17 June 2012. 2012; Vol. 97 (S1):Abstract no: 0927.
-
- Pennell DJ, Porter JB, Piga A, Lai Y, El‐Beshlawi A, Beloul K, et al. A multicenter, randomized, open‐label trial evaluating deferasirox compared with deferoxamine for the removal of cardiac iron in patients with beta‐thalassemia major and iron overload (CORDELIA). Blood. 2012; Vol. 121 (21).
Pepe 2013 {published data only}
-
- Pepe A, Meloni A, Pepe P, Capra M, D'Ascola DG, Santodirocco M, et al. Prospective comparison on cardiac and hepatic iron and cardiac function by MR in thalassemia major patients treated with combination deferiprone‐desferrioxamine versus deferiprone and desferrioxamine in monotherapy [abstract]. Blood 2011;118(21):Abstract.
-
- Pepe A, Meloni A, Rossi G, Cuccia L, D'Ascola GD, Santodirocco M, et al. Cardiac and hepatic iron and ejection fraction in thalassemia major: multicentre prospective comparison of combined deferiprone and deferoxamine therapy against deferiprone or deferoxamine monotherapy. http://www.jcmr‐online.com/content/pdf/1532‐429X‐15‐1.pdf (accessed 01 Augsut 2013). [DOI: 10.1186/1532-429X-15-1] - DOI - PMC - PubMed
-
- Pepe A, Meloni A, Rossi G, Ruffo GB, D'Ascola DG, Santodirocco M, et al. Cardiac iron and function by CMR in thalassemia major patients treated with combined deferiprone and desferrioxamine regimen versus montherapies: a multi‐center, observational and prospective study. European Heart Journal 2012;33:805.
-
- Pepe A, Rossi G, Meloni A, Dell'Amico MC, Capra M, Caruso V, et al. Prospective comparison on cardiac iron and liver iron by MR in thalassemia major patients treated with combination deferiprone‐desferrioxamine versus deferiprone and desferrioxamine in monotherapy [abstract]. Blood 2010;116(21):Abstract no: 5164.
-
- Pepe A, Rossi G, Meloni A, Dell'Amico MC, Capra M, Caruso V, et al. Prospective comparison on cardiac iron and liver iron by MR in thalassemia major patients treated with combination deferiprone‐desferrioxamine versus deferiprone and desferrioxamine in monotherapy [abstract]. Haematologica, Proceedings of the 15th Congress of the European Hematology Association, Barcelona, Spain 2010;95 Suppl 2:696, Abstract no: 1797.
Unal 2009 {published data only}
-
- Unal S, Hazirolan T, Beton B, Karabulut E, Gumruk F. The cardiac effects of desferoxamine deferiprone combination therapy and desferoxamine monotherapy in thalassemic patients [abstract]. Haematologica 2009;94 Suppl 2:514‐5, Abstract no: 1295.
References to ongoing studies
IRCT201110087677N1 {published data only}
-
- IRCT20110087677N1. The comparative study of incidence of lens opacity between Osfereal and Deferoxamine in major thalassemia. http://apps.who.int/trialsearch/trial.aspx?trialid=IRCT201110087677N1 (accessed 18 June 2012).
IRCT201206289827N2 {published data only}
-
- IRCT201206289827N2. Comparison of two methods of administration of deferoxamine (intravenous and subcutaneous) in terms of impact on reducing iron overload in thalassemia patients who have suffered heart failure. http://apps.who.int/trialsearch/trial.aspx?trialid=IRCT201206289827N2 (accessed 5 March 2013).
NCT01511848 {published data only}
-
- NCT01511848. Study of efficacy, safety of combined deferasirox and deferiprone versus combined deferiprone and desferal in conditions of iron overload. http://www.clinicaltrials.gov/ct2/show/NCT01511848?term=NCT01511848&... (accessed 18 June 2012).
Additional references
Agarwal 1992
-
- Agarwal MB, Gupte SS, Viswanathan C, Vasandani D, Ramanathan J, Desai N, et al. Long term assessment of efficacy and safety of L1, an oral iron chelator in transfusion‐dependent thalassaemia: Indian trial. British Journal of Haematology 1992;82(2):460‐6. - PubMed
Argyropoulou 2003
-
- Argyropoulou MI, Kiortsis DN, Efremidis SC. MRI of the liver and the pituitary gland in patients with beta‐thalassemia major: does hepatic siderosis predict pituitary iron deposition?. European Radiology 2003;13(1):12‐6. - PubMed
Aydinok 1999
-
- Aydinok Y, Nisli G, Kavakli K, Coker C, Kantar M, Cetingul N. Sequential use of deferiprone and desferrioxamine in primary school children with thalassaemia major in Turkey. Acta Haematologica 1999;102(1):17‐21. - PubMed
Bacon 1983
Berdoukas 2000
-
- Berdoukas V, Bohane T, Eagle C, Lindeman R, DeSilva K, Tobias V, et al. The Sydney Children's Hospital experience with the oral iron chelator deferiprone (L1). Transfusion Science 2000;23(3):239‐40. - PubMed
BNF 2012
-
- Joint Formulary Committee. British National Formulary. http://www.bnf.org. London: BMJ Group and Pharmaceutical Press <http://www.bnf.org> [Accessed on 14 May 2012], (accessed 14 May 2012).
BorgnaPignatti 1998a
-
- Borgna‐Pignatti C, Rugolotto S, Stefano P, Piga A, Gregorio F, Gamberini MR, et al. Survival and disease complications in thalassemia major. Annals of the New York Academy of Sciences 1998;850:227‐31. - PubMed
BorgnaPignatti 1998b
-
- Borgna‐Pignatti C, Franchini M, Gandini G, Vassanelli A, Gironcoli M, Aprili G. Subcutaneous bolus injection of deferoxamine in adult patients affected by onco‐hematologic diseases and iron overload. Haematologica 1998;83(9):788‐90. - PubMed
Bousquet 1983
-
- Bousquet J, Navarro M, Robert G, Aye P, Michel FB. Rapid desensitisation for desferrioxamine anaphylactoid reaction. Lancet 1983;2(8354):859‐60. - PubMed
Brittenham 1988
-
- Brittenham GM, Nienhuis AW. Desferrioxsamine use protects against heart disease and death from transfusional iron overload in thalassemia major [abstract]. Blood 1988;Suppl:56a.
Brittenham 1994
-
- Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. New England Journal of Medicine 1994;331(9):567‐73. - PubMed
Brittenham 2003a
-
- Brittenham GM, Nathan DG, Olivieri NF, Porter JB, Pippard M, Vichinsky EP, et al. Deferiprone and hepatic fibrosis. Blood 2003;101(12):5089‐90. - PubMed
Ceci 2002
-
- Ceci A, Felisi M, Catapano M, Baiardi P, Cipollina L, Ravera S, et al. The safety and effectiveness of deferiprone in a large‐scale, 3‐year study in Italian patients. British Journal of Haematology 2002;118(1):330‐6. - PubMed
Cohen 2000
-
- Cohen AR, Galanello R, Piga A, Dipalma A, Vullo C, Tricta F. Safety profile of the oral iron chelator: a multicentre study. British Journal of Haematology 2000;108(2):305‐12. - PubMed
Cohen 2003
-
- Cohen AR, Galanello R, Piga A, DeSanctis V, Tricta F. Safety and effectiveness of long term therapy with the oral iron chelator deferiprone profile of the oral iron chelator: a multicentre study. Blood 2003;102(5):1583‐7. - PubMed
Davis 2000
-
- Davis BA, Porter JB. Long‐term outcome of continuous 24‐hour deferoxamine infusion via indwelling intravenous catheters in high‐risk beta‐thalassemia. Blood 2000;95(4):1229‐36. - PubMed
De Sanctis 1996
-
- Sanctis V, Pinamonti A, Palma A, Sprocati M, Atti G, Gamberini MR, et al. Growth and development in thalassaemia major patients with severe bone lesions due to desferrioxamine. European Journal of Paediatrics 1996;155(5):368‐72. - PubMed
Del Vecchio 2000
-
- Vecchio GC, Crollo E, Schettini F, Fischer R, Mattia D. Factors influencing effectiveness of deferiprone in a thalassaemia major clinical setting. Acta Haematologica 2000;104(2‐3):99‐102. - PubMed
Ehlers 1991
-
- Ehlers KH, Giardina PJ, Leser ML, Engle MA, Hilgartner MW. Prolonged survival in patients with beta‐thalassemia major treated with desferrioxamine. Journal of Paediatrics 1991;118(4 Pt 1):540‐5. - PubMed
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
European Medicines Agency 2012
-
- European Medicines Agency (EMA). Exjade: EPAR. http://www.emea.europa.eu/docs/en_GB/document_library/PIP_decision/WC500... (accessed 14 May 2012).
FDA 2011
-
- US Food, Drug Administration. Oncologic Drugs Advisory Committee Briefing Document NDA # 21‐825: Ferriprox® (deferiprone) is an iron chelator indicated for the treatment of patients with transfusional iron overload when current chelation therapy is inadequate. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMateria... Acccessed 14 September 2011.
Fischer 2003
-
- Fischer R, Longo F, Nielsen P, Engelhardt R, Hider RC, Piga A. Monitoring long‐term efficacy of iron chelation therapy by deferiprone and desferrioxamine in patients with thalassaemia major: applicability of SQUID biomagnetic liver susceptometry. British Journal of Haematology 2003;121(6):938‐48. - PubMed
Fisher 2013
Franchini 2000
-
- Franchini M, Gandini G, Gironcoli M, Vassanelli A, Borgna‐Pignatti C, Aprili G. Safety and efficacy of subcutaneous bolus injection of deferoxamine in adult patients with iron overload. Blood 2000;95(9):2776‐9. - PubMed
Freedman 1990
-
- Freedman MH, Grisaru D, Olivieri N, MacLusky I, Thorner PS. Pulmonary syndrome in patients with thalassemia major receiving intravenous deferoxamine infusions. American Journal of Diseases of Children 1990;144(5):565‐9. - PubMed
Gabutti 1996
-
- Gabutti V, Piga A. Results of long‐term iron‐chelating therapy. Acta Haematologica 1996;95(1):26‐36. - PubMed
Hershko 1998
-
- Hershko C, Konijn AM, Link G. Iron chelators for thalassaemia. British Journal of Haematology 1998;101(3):399‐406. - PubMed
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration. Wiley ‐ Blackwell, 2011; Vol. Available from www.cochrane‐handbook.org.
Hoffbrand 1998
-
- Hoffbrand AV, AL‐Refaie F, Davis B, Siritanakatkul N, Jackson BF, Cochrane J, et al. Long‐term trial of deferiprone in 51 transfusion‐dependent iron overloaded patients. Blood 1998;91(1):295‐300. - PubMed
Kolnagou 2009
-
- Kolnagou A, Yazman D, Economides C, Kontoghiorghes GJ. Uses and limitations of serum ferritin, magnetic resonance imaging T2 and T2* in the diagnosis ofiron overload and in the ferrikinetics of normalization of the iron stores in thalassemia using the International Committee on Chelation deferiprone/deferoxamine combination protocol. Hemoglobin 2009;33(5):312‐22. - PubMed
Kontoghiorghes 1990
-
- Kontoghiorghes GJ, Bartlett AN, Hoffbrand AV, Goddard JG, Sheppard L, Barr J, et al. Long term trial with the oral iron chelator 1,2‐dimethyl‐3‐hydroxypyrid‐4‐1 (L1), I: iron chelation and metabolic studies. British Journal of Haematology 1990;76(2):295‐300. - PubMed
Koren 1989
-
- Koren G, Bentur Y, Strong D, Harvey E, Klein J, Baumal R, et al. Acute changes in renal function associated with DFO therapy. American Journal of Diseases of Children 1989;143(9):1077‐80. - PubMed
Koren 1991
-
- Koren G, Kochavi Atiya Y, Bentur Y, Olivieri NF. The effects of subcutaneous deferoxamine administration on renal function in thalassemia major. International Journal of Hematology 1991;54(5):371‐5. - PubMed
Kushner 2001
-
- Kushner JP, Porter JP, Olivieri NF. Secondary iron overload. Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. Education Program 2001;2001(1):47‐61. - PubMed
Lucas 2002
-
- Lucas GN, Perera BJ, Fonseka EA, Silva DD, Fernandopulle M, Karunatilaka DH, et al. Experience with the oral iron chelator deferiprone in transfusion‐dependent children. Ceylon Medical Journal 2002;47(4):119‐21. - PubMed
Maggio 2007
-
- Maggio A. Light and shadows in the iron chelation treatment of haematological diseases. British Journal of Haematology 2007;138(4):407‐21. - PubMed
Mazza 1998
-
- Mazza P, Amurri B, Lazzari G, Masi C, Palazzo G, Spartera MA, et al. Oral iron chelating therapy. A single center interim report on deferiprone (L1) in thalassemia. Haematologica 1998;83(6):496‐501. - PubMed
McLeod 2009
-
- McLeod C, Fleeman N, Kirkham J, Bagust A, Boland A, Chu P, et al. Deferasirox for the treatment of iron overload associated with regular blood transfusions (transfusional haemosiderosis) in patients suffering with chronic anaemia: a systematic review and economic evaluation. Health Technology Assessment 2009;13(1):iii‐iv, ix‐xi, 1‐121. - PubMed
Meehpohl 2012
Miller 1981
-
- Miller KB, Rosenwasser LJ, Bessette JM, Beer DJ, Rocklin RE. Rapid desenstitisation for desferrioxamine anaphylactic reaction (letter). Lancet 1981;1(8228):1059. - PubMed
Modell 2000
-
- Modell B, Khan M, Darlison M. Survival in beta‐thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet 2000;355(9220):2051‐2. - PubMed
Nathan 2002
-
- Nathan DG, Weatherall DJ. Academic freedom in clinical research. New England Journal of Medicine 2002;347(17):1368‐71. - PubMed
Neufeld 2006
O'Brien 1974
-
- O'Brien RT. Ascorbic acid enhancement of desferrioxamine‐induced urinary iron excretion in thalassaemia major. Annals New York Academy of Science 1974;232:221‐5. - PubMed
Olivieri 1994
-
- Olivieri NF, Nathan DG, MacMillan JH, Wayne AS, Liu PP, McGee A, et al. Survival in medically treated patients with homozygous beta‐thalassemia. New England Journal of Medicine 1994;331(9):574‐8. - PubMed
Olivieri 1995a
-
- Olivieri NF, Brittenham GM, Matsui D, Berkovitch M, Blendis LM, Cameron RG, et al. Iron‐chelation therapy with oral deferiprone in patients with thalassemia major. New England Journal of Medicine 1995;332(14):918‐22. - PubMed
Olivieri 1995b
-
- Olivieri NF, Brittenham GM, Armstrong SAM, Barsran RK, Daneman R, Daneman N, et al. First prospective randomized trial of the iron chelators deferiprone and deferoxamine [abstract]. Blood 1995;86 Suppl 1:249a.
Olivieri 1997a
-
- Olivieri NF, Brittenham GM. Final results of the randomized trial of deferiprone (L1) and deferoxamine (DFO) [abstract]. Blood 1997;90 Suppl 1:264a.
Olivieri 1997b
-
- Olivieri NF, Brittenham GM. Iron‐chelating therapy and the treatment of thalassemia. Blood 1997;89(3):739‐61. - PubMed
Olivieri 1998
-
- Olivieri N, Brittenham GM, McLaren CE, Templeton DM, Cameron RG, McClelland RA, et al. Long‐term safety and effectiveness of iron‐chelation therapy with deferiprone for thalassemia major. New England Journal of Medicine 1998;339(7):417‐23. - PubMed
Olivieri 1999
-
- Olivieri NF. The beta‐thalassemias. New England Journal of Medicine 1999;341(2):99‐109. - PubMed
Pati 1999
-
- Pati HP, Choudry VP. Deferiprone (L1) associated neutropenia in beta thalassaemia major: an Indian experience. European Journal of Haematology 1999;63(4):267‐8. - PubMed
Pippard 1978a
-
- Pippard MJ, Letsky EA, Callender ST, Weatherall DJ. Prevention of iron loading in transfusion‐dependent thalassaemia. Lancet 1978;1(8075):1178‐81. - PubMed
Pippard 1979
-
- Pippard MJ, Callender ST, Warner GT, Weatherall DJ. Iron absorption and loading in beta‐thalassaemia intermedia. Lancet 1979;2(8147):819‐21. - PubMed
Pippard 2000
-
- Pippard MJ, Weatherall DJ. Oral iron chelation therapy for thalassaemia: an uncertain scene. British Journal of Haematology 2000;111(1):2‐5. - PubMed
Pootrakul 1988
-
- Pootrakul P, Kitcharoen K, Yansukon P, Wasi P, Fucharoen S, Charoenlarp P, et al. The effect of erythroid hyperplasia on iron balance. Blood 1988;71(4):1124‐9. - PubMed
Porter 1989
-
- Porter J, Huehns E. The toxic effects of desferrioxamine. Balliere's Clinical Haematology 1989;2(2):459‐74. - PubMed
Porter 2002
-
- Porter J, Davies BA. Monitoring chelation therapy to achieve optimal outcome in the treatment of thalassaemia. Best Practice Research in Clinical Haematology 2002;15(2):329‐68. - PubMed
Propper 1976
-
- Propper RD, Shurin SB, Nathan DG. Reassessment of the use of desferrioxamine B in iron overload. New England Journal of Medicine 1976;294(26):1421‐3. - PubMed
Review Manager 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Richardson 1993
-
- Richardson ME, Matthews RN, Alison JF, Menahem S, Mitvalsky J, Byrt E, et al. Prevention of heart disease by subcutaneous desferrioxamine in patients with thalassaemia major. Australian and New Zealand Journal of Medicine 1993;23(6):656‐61. - PubMed
Robins‐Browne 1985
Rombos 2000
-
- Rombos Y, Tzanetea R, Konstantopoulos K, Simitizis S, Zervas C, Kyriaki P, et al. Chelation therapy in patients with thalassemia using the orally active iron chelator deferiprone (L1). Haematologica 2000;85(2):115‐7. - PubMed
Tenenbein 1992
-
- Tenenbein M, Kowalski S, Sienko A, Bowden DH, Adamson IYR. Pulmonary toxic effects of continuous desferrioxamine administration in acute iron poisoning. Lancet 1992;339(8795):699‐701. - PubMed
Tondury 1990
-
- Tondury P, Kontoghiorghes GJ, Ridolfi‐Luthy A, Hirt A, Hoffbrand AV, Lottenbach AM, et al. L1 (1,2‐dimethyl‐3‐hydroxypyrid‐4‐one) for oral iron chelation in patients with beta‐thalassaemia major. British Journal of Haematology 1990;76(4):550‐3. - PubMed
Tondury 1998
-
- Tondury P, Zimmermann A, Nielsen P, Hirt A. Liver iron and fibrosis during long‐term treatment with deferiprone in Swiss thalassaemic patients. British Journal of Haematology 1998;101(3):413‐5. - PubMed
Viens 2004
Wanless 2002
-
- Wanless IR, Sweeney G, Dhillon AP, Piga A, Galanello R, Gamberini MR, et al. Lack of progressive hepatic fibrosis during long‐term therapy with deferiprone in subjects with transfusion‐dependent beta‐thalasssemia. Blood 2002;100(5):1566‐9. - PubMed
Wapnick 1969
-
- Wapnick AA, Lynch SR, Charlton RW, Seftel HC, Bothwell TH. The effect of ascorbic acid deficiency on desferrioxamine‐induced urinary iron excretion. British Journal of Haematology 1969;17(6):563‐8. - PubMed
Weatherall 2001a
-
- Weatherall DJ, Clegg JB. The Thalassamia Syndromes. 4th Edition. Oxford: Blackwell Sciences Ltd, 2001.
Weatherall 2001b
-
- Weatherall DJ. Phenotype‐genotype relationships in monogenic disease: lessons from the thalassaemias. Nature Reviews. Genetics 2001;2(4):245‐55. - PubMed
Wolfe 1985
-
- Wolfe L, Olivieri N, Sallan D, Colan S, Rose V, Propper R, et al. Prevention of cardiac disease by subcutaneous deferoxamine in patients with thalassemia major. New England Journal of Medicine 1985;312(25):1600‐3. - PubMed
Zurlo 1989
-
- Zurlo MG, DeStefano P, Borgna‐Pignatti C, Palma A, Piga A, Melevendi C, et al. Survival and causes of death in thalassemia major. Lancet 1989;2(8653):27‐30. - PubMed
References to other published versions of this review
Roberts 2003
Roberts 2005
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

