Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding
- PMID: 23964100
- PMCID: PMC4033279
- DOI: 10.1136/gutjnl-2013-305008
Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding
Abstract
Objectives: Roux-en-Y gastric bypass (RYGB) has greater efficacy for weight loss in obese patients than gastric banding (BAND) surgery. We hypothesise that this may result from different effects on food hedonics via physiological changes secondary to distinct gut anatomy manipulations.
Design: We used functional MRI, eating behaviour and hormonal phenotyping to compare body mass index (BMI)-matched unoperated controls and patients after RYGB and BAND surgery for obesity.
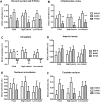
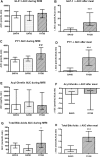
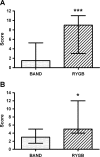
Results: Obese patients after RYGB had lower brain-hedonic responses to food than patients after BAND surgery. RYGB patients had lower activation than BAND patients in brain reward systems, particularly to high-calorie foods, including the orbitofrontal cortex, amygdala, caudate nucleus, nucleus accumbens and hippocampus. This was associated with lower palatability and appeal of high-calorie foods and healthier eating behaviour, including less fat intake, in RYGB compared with BAND patients and/or BMI-matched unoperated controls. These differences were not explicable by differences in hunger or psychological traits between the surgical groups, but anorexigenic plasma gut hormones (GLP-1 and PYY), plasma bile acids and symptoms of dumping syndrome were increased in RYGB patients.
Conclusions: The identification of these differences in food hedonic responses as a result of altered gut anatomy/physiology provides a novel explanation for the more favourable long-term weight loss seen after RYGB than after BAND surgery, highlighting the importance of the gut-brain axis in the control of reward-based eating behaviour.
Keywords: Bile Acid; Brain Imaging; Brain/Gut Interaction; Gastrointestinal Hormones; Obesity Surgery.
Figures





Comment in
-
Does the gut-brain axis control anticipatory food reward? Novel insights from bariatric surgery.Gut. 2014 Jun;63(6):868-9. doi: 10.1136/gutjnl-2013-305488. Epub 2013 Aug 29. Gut. 2014. PMID: 23990629 No abstract available.
References
-
- Sjostrom L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012;307:56–65 - PubMed
-
- Dixon JB, le Roux CW, Rubino F, et al. Bariatric surgery for type 2 diabetes. Lancet 2012;379:2300–11 - PubMed
-
- Rubino F, Schauer PR, Kaplan LM, et al. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med 2010;61:393–411 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
