NR2B subunit of the NMDA glutamate receptor regulates appetite in the parabrachial nucleus
- PMID: 23964123
- PMCID: PMC3767546
- DOI: 10.1073/pnas.1314137110
NR2B subunit of the NMDA glutamate receptor regulates appetite in the parabrachial nucleus
Erratum in
-
Correction for Wu et al., NR2B subunit of the NMDA glutamate receptor regulates appetite in the parabrachial nucleus.Proc Natl Acad Sci U S A. 2025 Feb 4;122(5):e2425524121. doi: 10.1073/pnas.2425524121. Epub 2025 Jan 9. Proc Natl Acad Sci U S A. 2025. PMID: 39786924 Free PMC article. No abstract available.
Abstract
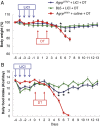
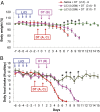
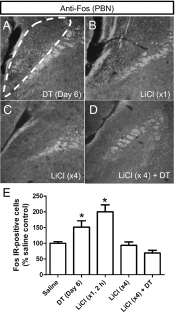
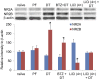
Diphtheria toxin-mediated, acute ablation of hypothalamic neurons expressing agouti-related protein (AgRP) in adult mice leads to anorexia and starvation within 7 d that is caused by hyperactivity of neurons within the parabrachial nucleus (PBN). Because NMDA glutamate receptors are involved in various synaptic plasticity-based behavioral modifications, we hypothesized that modulation of the NR2A and NR2B subunits of the NMDA receptor in PBN neurons could contribute to the anorexia phenotype. We observed by Western blot analyses that ablation of AgRP neurons results in enhanced expression of NR2B along with a modest suppression of NR2A. Interestingly, systemic administration of LiCl in a critical time window before AgRP neuron ablation abolished the anorectic response. LiCl treatment suppressed NR2B levels in the PBN and ameliorated the local Fos induction that is associated with anorexia. This protective role of LiCl on feeding was blunted in vagotomized mice. Chronic infusion of RO25-6981, a selective NR2B inhibitor, into the PBN recapitulated the role of LiCl in maintaining feeding after AgRP neuron ablation. We suggest that the accumulation of NR2B subunits in the PBN contributes to aphagia in response to AgRP neuron ablation and may be involved in other forms of anorexia.
Keywords: NR2B signaling; feeding behavior; nausea.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Paoletti P, Neyton J. NMDA receptor subunits: Function and pharmacology. Curr Opin Pharmacol. 2007;7(1):39–47. - PubMed
-
- Hung CY, Covasa M, Ritter RC, Burns GA. Hindbrain administration of NMDA receptor antagonist AP-5 increases food intake in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;290(3):R642–R651. - PubMed
-
- Burns GA, Ritter RC. The non-competitive NMDA antagonist MK-801 increases food intake in rats. Pharmacol Biochem Behav. 1997;56(1):145–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

