Diagnostic accuracy of a new point-of-care screening assay for celiac disease
- PMID: 23964145
- PMCID: PMC3746383
- DOI: 10.3748/wjg.v19.i31.5111
Diagnostic accuracy of a new point-of-care screening assay for celiac disease
Abstract
Aim: To determine the diagnostic accuracy of a new point-of-care assay detecting anti-deamidated gliadin peptides in celiac disease (CD) patients.
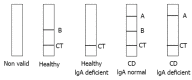
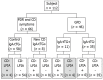
Methods: One-hundred-and-twelve patients (age range: 1.8-79.2 years old) with clinical symptoms suggestive of CD and/or first-degree relatives (FDR) of CD patients (n = 66), and confirmed CD on a gluten-free diet (GFD) (n = 46), were prospectively enrolled in the study at Gastroenterology outpatient clinics for adult patients and from the Gastroenterology Consultation Ward at the Pediatric Department of the University Hospital of Geneva. Written informed consent was obtained from all subjects enrolled. The study received approval from the local ethics committee. The original CD diagnosis had been based on serum-positive IgA anti-tissue transglutaminase enzyme-linked immunosorbent assay (ELISA) (QuantaLite™, Inova Diagnostics, San Diego, CA, United States) and on biopsy results. Serum samples from all study participants were tested by the new CD lateral flow immunochromatographic assay (CD-LFIA) device, Simtomax® Blood Drop (Augurix SA, BioArk, Monthey, Switzerland) to detect immunoglobulin (Ig)A and IgG antibodies against deamidated gliadin peptides. The diagnostic performance was evaluated using receiver operating characteristic curves with 95%CIs. A cut-off of 2 on the Rann colorimetric scale was used to calculate the device's sensitivity and specificity.
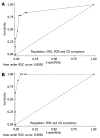
Results: CD-LFIA was highly accurate in detecting untreated celiac patients. In the group of patients with CD symptoms and/or FDR, eight new cases of CD were detected by ELISA and biopsy. All of these new cases were also correctly identified by CD-LFIA. The test yielded four false positive and four false negative results. The false positive results were all within the groups with clinical symptoms suggestive of CD and/or FDR, whereas the false negative results were all within the GFD group. The test yeld a sensitivity of 78.9% (95%CI: 54.4-93.9) and specificity of 95.7% (95%CI: 89.4-98.8), and the area under the curve reached 0.893 (95%CI: 0.798-0.988). The Kappa coefficient, calculated according to the values obtained by two readers from the same device, was of 0.96 (SE: 0.06). When the GFD patients were excluded from the analysis, the area under the curve reached 0.989 (95%CI: 0.971-1.000) and the Kappa coefficient, calculated according to the values obtained by two readers from the same device, became 0.96 (SE: 0.07). Furthermore, using the Rann scale cut-off of 2 without the GFD patients, sensitivity was 100% and specificity was 93.1% (95%CI: 83.3-98.1).
Conclusion: The new CD-LFIA rapid screening test shows good diagnostic accuracy, sensitivity and specificity, and may rule out CD in patients with CD-related symptoms.
Keywords: Celiac disease; Deamidated gliadin; Point-of-care assay; Screening; Total immunoglobulin A.
Figures
Similar articles
-
Early diagnosis of celiac disease in IgA deficient children: contribution of a point-of-care test.BMC Gastroenterol. 2014 Nov 6;14:186. doi: 10.1186/1471-230X-14-186. BMC Gastroenterol. 2014. PMID: 25376178 Free PMC article.
-
Evaluation of a point-of-care test based on deamidated gliadin peptides for celiac disease screening in a large pediatric population.Eur J Gastroenterol Hepatol. 2012 Dec;24(12):1418-23. doi: 10.1097/MEG.0b013e3283582d95. Eur J Gastroenterol Hepatol. 2012. PMID: 23032795
-
Diagnostic efficacy of the ELISA test for the detection of deamidated anti-gliadin peptide antibodies in the diagnosis and monitoring of celiac disease.J Clin Lab Anal. 2009;23(3):165-71. doi: 10.1002/jcla.20313. J Clin Lab Anal. 2009. PMID: 19455636 Free PMC article.
-
AGA Clinical Practice Update on Diagnosis and Monitoring of Celiac Disease-Changing Utility of Serology and Histologic Measures: Expert Review.Gastroenterology. 2019 Mar;156(4):885-889. doi: 10.1053/j.gastro.2018.12.010. Epub 2018 Dec 19. Gastroenterology. 2019. PMID: 30578783 Free PMC article. Review.
-
Tests for Serum Transglutaminase and Endomysial Antibodies Do Not Detect Most Patients With Celiac Disease and Persistent Villous Atrophy on Gluten-free Diets: a Meta-analysis.Gastroenterology. 2017 Sep;153(3):689-701.e1. doi: 10.1053/j.gastro.2017.05.015. Epub 2017 May 22. Gastroenterology. 2017. PMID: 28545781 Free PMC article. Review.
Cited by
-
The Role of an IgA/IgG-Deamidated Gliadin Peptide Point-of-Care Test in Predicting Persistent Villous Atrophy in Patients With Celiac Disease on a Gluten-Free Diet.Am J Gastroenterol. 2017 Dec;112(12):1859-1867. doi: 10.1038/ajg.2017.357. Epub 2017 Oct 10. Am J Gastroenterol. 2017. PMID: 29016564
-
The Profile of Urinary Headspace Volatile Organic Compounds After 12-Week Intake of Oligofructose-Enriched Inulin by Children and Adolescents with Celiac Disease on a Gluten-Free Diet: Results of a Pilot, Randomized, Placebo-Controlled Clinical Trial.Molecules. 2019 Apr 5;24(7):1341. doi: 10.3390/molecules24071341. Molecules. 2019. PMID: 30959740 Free PMC article. Clinical Trial.
-
A targeted metabolomic protocol for quantitative analysis of volatile organic compounds in urine of children with celiac disease.RSC Adv. 2018 Oct 29;8(64):36534-36541. doi: 10.1039/c8ra07342b. eCollection 2018 Oct 26. RSC Adv. 2018. PMID: 35558911 Free PMC article.
-
Differentiating coeliac disease from irritable bowel syndrome by urinary volatile organic compound analysis--a pilot study.PLoS One. 2014 Oct 16;9(10):e107312. doi: 10.1371/journal.pone.0107312. eCollection 2014. PLoS One. 2014. PMID: 25330367 Free PMC article.
-
Novel screening test for celiac disease using peptide functionalised gold nanoparticles.World J Gastroenterol. 2018 Dec 21;24(47):5379-5390. doi: 10.3748/wjg.v24.i47.5379. World J Gastroenterol. 2018. PMID: 30598582 Free PMC article.
References
-
- Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, Troncone R, Giersiepen K, Branski D, Catassi C, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–160. - PubMed
-
- Reeves GE, Squance ML, Duggan AE, Murugasu RR, Wilson RJ, Wong RC, Gibson RA, Steele RH, Pollock WK. Diagnostic accuracy of coeliac serological tests: a prospective study. Eur J Gastroenterol Hepatol. 2006;18:493–501. - PubMed
-
- Giersiepen K, Lelgemann M, Stuhldreher N, Ronfani L, Husby S, Koletzko S, Korponay-Szabó IR. Accuracy of diagnostic antibody tests for coeliac disease in children: summary of an evidence report. J Pediatr Gastroenterol Nutr. 2012;54:229–241. - PubMed
-
- Tursi A, Brandimarte G, Giorgetti G, Gigliobianco A, Lombardi D, Gasbarrini G. Low prevalence of antigliadin and anti-endomysium antibodies in subclinical/silent celiac disease. Am J Gastroenterol. 2001;96:1507–1510. - PubMed
-
- Vahedi K, Mascart F, Mary JY, Laberenne JE, Bouhnik Y, Morin MC, Ocmant A, Velly C, Colombel JF, Matuchansky C. Reliability of antitransglutaminase antibodies as predictors of gluten-free diet compliance in adult celiac disease. Am J Gastroenterol. 2003;98:1079–1087. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

