Secretory IgA: Designed for Anti-Microbial Defense
- PMID: 23964273
- PMCID: PMC3734371
- DOI: 10.3389/fimmu.2013.00222
Secretory IgA: Designed for Anti-Microbial Defense
Abstract
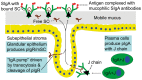
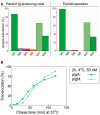
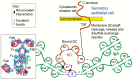
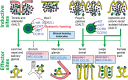
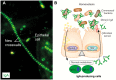
Prevention of infections by vaccination remains a compelling goal to improve public health. Mucosal vaccines would make immunization procedures easier, be better suited for mass administration, and most efficiently induce immune exclusion - a term coined for non-inflammatory antibody shielding of internal body surfaces, mediated principally by secretory immunoglobulin A (SIgA). The exported antibodies are polymeric, mainly IgA dimers (pIgA), produced by local plasma cells (PCs) stimulated by antigens that target the mucose. SIgA was early shown to be complexed with an epithelial glycoprotein - the secretory component (SC). A common SC-dependent transport mechanism for pIgA and pentameric IgM was then proposed, implying that membrane SC acts as a receptor, now usually called the polymeric Ig receptor (pIgR). From the basolateral surface, pIg-pIgR complexes are taken up by endocytosis and then extruded into the lumen after apical cleavage of the receptor - bound SC having stabilizing and innate functions in the secretory antibodies. Mice deficient for pIgR show that this is the only receptor responsible for epithelial export of IgA and IgM. These knockout mice show a variety of defects in their mucosal defense and changes in their intestinal microbiota. In the gut, induction of B-cells occurs in gut-associated lymphoid tissue, particularly the Peyer's patches and isolated lymphoid follicles, but also in mesenteric lymph nodes. PC differentiation is accomplished in the lamina propria to which the activated memory/effector B-cells home. The airways also receive such cells from nasopharynx-associated lymphoid tissue but by different homing receptors. This compartmentalization is a challenge for mucosal vaccination, as are the mechanisms used by the mucosal immune system to discriminate between commensal symbionts (mutualism), pathobionts, and overt pathogens (elimination).
Keywords: GALT; MALT; NALT; antibodies; commensals; germinal centers; mucosa; pathogens.
Figures




References
-
- Besredka A. De la vaccination contre les états typhoides par la voie buccale. Ann Inst Pasteur (1919) 33:882–903
-
- Davies A. An investigation into the serological properties of dysentery stools. Lancet (1922) 2:1009–12 10.1016/S0140-6736(01)16832-7 - DOI
-
- Besredka A. Local Immunization. Baltimore: Williams & Wilkins Company; (1927).
-
- Pierce AE. Specific antibodies at mucous surfaces. Vet Rev Annot (1959) 5:17–36
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

