A tale of two neglected systems-structure and function of the thin- and thick-walled sieve tubes in monocotyledonous leaves
- PMID: 23964280
- PMCID: PMC3734358
- DOI: 10.3389/fpls.2013.00297
A tale of two neglected systems-structure and function of the thin- and thick-walled sieve tubes in monocotyledonous leaves
Abstract
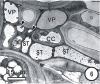
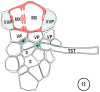
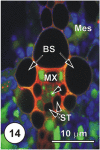
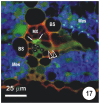
There is a large body of information relating to the ontogeny, development and the vasculature of eudicotyledonous leaves. However, there is less information available concerning the vascular anatomy of monocotyledonous leaves. This is surprising, given that there are two uniquely different phloem systems present in large groups such as grasses and sedges. Monocotyledonous leaves contain marginal, large, intermediate, and small longitudinal veins that are interconnected by numerous transverse veins. The longitudinal veins contain two metaphloem sieve tube types, which, based upon their ontogeny and position within the phloem, are termed early (thin-walled) and late (thick-walled) sieve tubes. Early metaphloem comprises sieve tubes, companion cells and vascular parenchyma (VP) cells, whilst the late metaphloem, contains thick-walled sieve tubes (TSTs) that lack companion cells. TSTs are generally adjacent to, or no more than one cell removed from the metaxylem. Unlike thin-walled sieve tube (ST) -companion cell complexes, TSTs are connected to parenchyma by pore-plasmodesma units and are generally symplasmically isolated from the STs. This paper addresses key structural and functional differences between thin- and thick-walled sieve tubes and explores the unique advantages of alternate transport strategies that this 5-7 million years old dual system may offer. It would seem that these two systems may enhance, add to, or play a significant role in increasing the efficiency of solute retrieval as well as of assimilate transfer.
Keywords: early and late metaphloem; monocotyledon; thick-walled and thin-walled sieve tubes; vascular bundle structure.
Figures














References
-
- Aoki N., Scofield G. N., Wang X. D., Patrick J. W., Offler C. E., Furbank R. T. (2004). Expression and localisation analysis of the wheat sucrose transporter TaSUT1 in vegetative tissues. Planta 219, 176–184 - PubMed
-
- Bosabalidis A. M., Evert R. F., Russin W. A. (1994). Ontogeny of the vascular bundles and contiguous tissues in the maize leaf blade. Am. J. Bot. 81, 745–752 10.2307/2445653 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

