Structural determinants of drug partitioning in surrogates of phosphatidylcholine bilayer strata
- PMID: 23964749
- PMCID: PMC3884577
- DOI: 10.1021/mp400204y
Structural determinants of drug partitioning in surrogates of phosphatidylcholine bilayer strata
Abstract
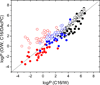
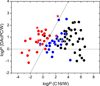
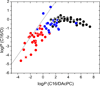
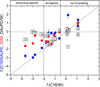
The knowledge of drug concentrations in bilayer headgroups, core, and at the interface between them is a prerequisite for quantitative modeling of drug interactions with many membrane-bound transporters, metabolizing enzymes and receptors, which have the binding sites located in the bilayer. This knowledge also helps understand the rates of trans-bilayer transport because balanced interactions of drugs with the bilayer strata lead to high rates, while excessive affinities for any stratum cause a slowdown. Experimental determination of bilayer location is so tedious and costly that the data are only available for some fifty compounds. To extrapolate these valuable results to more compounds at a higher throughput, surrogate phases have been used to obtain correlates of the drug affinities for individual strata. We introduced a novel system, consisting of a diacetyl phosphatidylcholine (DAcPC) solution with the water content of the fluid bilayer as the headgroup surrogate and n-hexadecane (C16) representing the core. The C16/DAcPC partition coefficients were measured for 113 selected compounds, containing structural fragments that are frequently occurring in approved drugs. The data were deconvoluted into the ClogP-based fragment solvation characteristics and processed using a solvatochromic correlation. Increased H-bond donor ability and excess molar refractivity of compounds promote solvation in the DAcPC phase as compared to bulk water, contrary to H-bond acceptor ability, dipolarity/polarizability, and volume. The results show that aromates have more balanced distribution in bilayer strata, and thus faster trans-bilayer transport, than similar alkanes. This observation is in accordance with the frequent occurrence of aromatic rings in approved drugs and with the role of rigidity of drug molecules in promoting intestinal absorption. Bilayer locations, predicted using the C16/DAcPC system, are in excellent agreement with available experimental data, in contrast to other surrogate systems.
Figures

Comment in
-
Comment on "structural determinants of drug partitioning in surrogates of phosphatidylcholine bilayer strata".Mol Pharm. 2015 Apr 6;12(4):1328-9. doi: 10.1021/mp500382h. Epub 2015 Mar 27. Mol Pharm. 2015. PMID: 25584416
-
Response to "comment on 'structural determinants of drug partitioning in surrogates of phosphatidylcholine bilayer strata'".Mol Pharm. 2015 Apr 6;12(4):1330-4. doi: 10.1021/acs.molpharmaceut.5b00139. Epub 2015 Mar 27. Mol Pharm. 2015. PMID: 25812003 Free PMC article.
Similar articles
-
Polarity of Hydrated Phosphatidylcholine Headgroups.Langmuir. 2019 Jun 25;35(25):8460-8471. doi: 10.1021/acs.langmuir.8b03992. Epub 2019 Jun 17. Langmuir. 2019. PMID: 31244216 Free PMC article.
-
Structure-based prediction of drug distribution across the headgroup and core strata of a phospholipid bilayer using surrogate phases.Mol Pharm. 2014 Oct 6;11(10):3577-95. doi: 10.1021/mp5003366. Epub 2014 Sep 18. Mol Pharm. 2014. PMID: 25179490 Free PMC article.
-
Partitioning of organic compounds in phases imitating the headgroup and core regions of phospholipid bilayers.Langmuir. 2006 Feb 14;22(4):1869-74. doi: 10.1021/la052187j. Langmuir. 2006. PMID: 16460120 Free PMC article.
-
Structure and functional properties of diacylglycerols in membranes.Prog Lipid Res. 1999 Jan;38(1):1-48. doi: 10.1016/s0163-7827(98)00021-6. Prog Lipid Res. 1999. PMID: 10396601 Review.
-
Molecular dynamics simulation studies of lipid bilayer systems.Acta Biochim Pol. 2000;47(3):601-11. Acta Biochim Pol. 2000. PMID: 11310963 Review.
Cited by
-
Polarity of Hydrated Phosphatidylcholine Headgroups.Langmuir. 2019 Jun 25;35(25):8460-8471. doi: 10.1021/acs.langmuir.8b03992. Epub 2019 Jun 17. Langmuir. 2019. PMID: 31244216 Free PMC article.
-
Scalable Synthesis and Purification of Acetylated Phosphatidyl Choline Headgroup.Org Process Res Dev. 2017 Feb 17;21(2):177-181. doi: 10.1021/acs.oprd.6b00261. Epub 2017 Jan 24. Org Process Res Dev. 2017. PMID: 30792570 Free PMC article.
-
Response to "comment on 'structural determinants of drug partitioning in surrogates of phosphatidylcholine bilayer strata'".Mol Pharm. 2015 Apr 6;12(4):1330-4. doi: 10.1021/acs.molpharmaceut.5b00139. Epub 2015 Mar 27. Mol Pharm. 2015. PMID: 25812003 Free PMC article.
-
Drug Distribution. Part 1. Models to Predict Membrane Partitioning.Pharm Res. 2017 Mar;34(3):535-543. doi: 10.1007/s11095-016-2085-z. Epub 2016 Dec 15. Pharm Res. 2017. PMID: 27981450 Free PMC article.
-
Conformations of Macrocyclic Peptides Sampled by Nuclear Magnetic Resonance: Models for Cell-Permeability.J Am Chem Soc. 2023 Dec 20;145(50):27601-27615. doi: 10.1021/jacs.3c09367. Epub 2023 Dec 7. J Am Chem Soc. 2023. PMID: 38062770 Free PMC article.
References
-
- Seelig A, Gatlik-Landwojtowicz E. Inhibitors of multidrug efflux transporters: Their membrane and protein interactions. Mini-Rev. Med. Chem. 2005;5:135–151. - PubMed
-
- Luong C, Miller A, Barnett J, Chow J, Ramesha C, Browner MF. Flexibility of the NSAID binding site in the structure of human cyclooxygenase-2. Nat. Struct. Biol. 1996;3:927–933. - PubMed
-
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982;157:105–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

