Protocol for a double-blind randomised controlled trial of low dose intradermal grass pollen immunotherapy versus a histamine control on symptoms and medication use in adults with seasonal allergic rhinitis (PollenLITE)
- PMID: 23965180
- PMCID: PMC3765857
- DOI: 10.1186/2045-7022-3-27
Protocol for a double-blind randomised controlled trial of low dose intradermal grass pollen immunotherapy versus a histamine control on symptoms and medication use in adults with seasonal allergic rhinitis (PollenLITE)
Abstract
Background: Subcutaneous immunotherapy with high dose grass pollen (typically microgram quantities) was first described over 100 years ago. This treatment suppresses allergen-induced cutaneous late responses, with lesser effects on early responses. We previously reported that repeated 2-weekly intradermal injections of grass pollen - containing approximately 7 ng of major allergen Phl p 5 - led to a progressive suppression of the allergen-induced cutaneous response, and that by the sixth injection, this was inhibited by over 90%. The purpose of this trial is to investigate the clinical efficacy of intradermal desensitisation with low doses (i.e. nanogram quantities) of grass pollen allergen for seasonal allergic rhinitis.
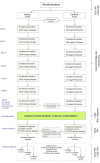
Methods/design: The Pollen Low dose Intradermal therapy Evaluation (PollenLITE) is a single centre double-blind randomised parallel group controlled trial of the efficacy and safety of intradermal grass pollen injections plus standard treatment, versus histamine injections plus standard treatment, in adults with moderate-severe grass pollen-induced allergic rhinitis ('summer hay fever'). A minimum of ninety adults with a history of moderate-severe persistent allergic rhinitis during the UK grass pollen season will be randomised into two equal groups to receive 7 or 8 intradermal injections of grass pollen extract (containing approximately 7 ng of major allergen Phl p 5) or histamine, before the grass pollen season. In the summer, participants will score their symptoms, medication requirements, visual analogue scores, and complete EuroQOL (EQ-5D-5 L) and mini Rhinoconjunctivitis Quality of Life Questionnaires. Global assessments will also be recorded at the end of the pollen season. Blood samples will be collected from all participants for mechanistic immune assays. Skin punch biopsies will also be collected in 40 participants selected at random from intradermal injection sites after the grass pollen season for mechanistic assays. Finally, to investigate if the desensitising effect of intradermal immunotherapy on cutaneous responses is long-lasting, all participants will be randomised to receive a follow up intradermal injection after 3, 6 or 12 months with measurement of early and late response sizes.
Discussion: Randomisation began in February 2013 and the final participant will complete the trial protocol in August 2014.
Trial registration: ISRCTN 78413121EudraCT number 2012-002193-31.
Figures
References
-
- Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;1:1572–1573.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources