Structural characterization of new microcystins containing tryptophan and oxidized tryptophan residues
- PMID: 23966035
- PMCID: PMC3766880
- DOI: 10.3390/md11083025
Structural characterization of new microcystins containing tryptophan and oxidized tryptophan residues
Abstract
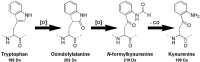
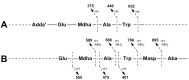
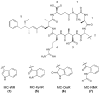
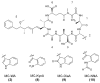
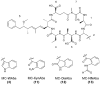
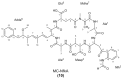
Microcystins are cyclic peptides produced by cyanobacteria, which can be harmful to humans and animals when ingested. Eight of the (more than) 90 microcystin variants presently characterized, contain the amino acid tryptophan. The well-researched oxidation products of tryptophan; kynurenine, oxindolylalanine, and N-formylkynurenine, have been previously identified in intact polypeptides but microcystin congeners containing oxidized tryptophan moieties have not been reported. Liquid chromatography-tandem mass spectrometric analysis of an extract of Microcystis CAWBG11 led to the tentative identification of two new tryptophan-containing microcystins (MC‑WAba and MC-WL), as well as eight other microcystin analogs containing kynurenine, oxindolylalanine and N‑formylkynurenine (Nfk). Investigation of one of these congeners (MC‑NfkA) by nuclear magnetic resonance spectroscopy was used to verify the presence of Nfk in the microcystin. Liquid chromatography-mass spectrometry analysis of a tryptophan oxidation experiment demonstrated that tryptophan-containing microcystins could be converted into oxidized tryptophan analogs and that low levels of oxidized tryptophan congeners were present intracellularly in CAWBG11. MC-NfkR and MC-LNfk were detected in standards of MC-WR and MC-LW, indicating that care during storage of tryptophan-containing microcystins is required.
Figures
Similar articles
-
High levels of structural diversity observed in microcystins from Microcystis CAWBG11 and characterization of six new microcystin congeners.Mar Drugs. 2014 Nov 13;12(11):5372-95. doi: 10.3390/md12115372. Mar Drugs. 2014. PMID: 25402827 Free PMC article.
-
Identification of microcystin toxins from a strain of Microcystis aeruginosa by liquid chromatography introduction into a hybrid linear ion trap-Fourier transform ion cyclotron resonance mass spectrometer.Anal Chem. 2006 Jan 15;78(2):501-12. doi: 10.1021/ac051556d. Anal Chem. 2006. PMID: 16408933
-
Novel Microcystins from Planktothrix prolifica NIVA-CYA 544 Identified by LC-MS/MS, Functional Group Derivatization and 15N-labeling.Mar Drugs. 2019 Nov 15;17(11):643. doi: 10.3390/md17110643. Mar Drugs. 2019. PMID: 31731697 Free PMC article.
-
Account: characterization and identification of microcystins by mass spectrometry.Eur J Mass Spectrom (Chichester). 2014;20(1):1-19. doi: 10.1255/ejms.1250. Eur J Mass Spectrom (Chichester). 2014. PMID: 24881451 Review.
-
Tripping up Trp: Modification of protein tryptophan residues by reactive oxygen species, modes of detection, and biological consequences.Free Radic Biol Med. 2015 Dec;89:220-8. doi: 10.1016/j.freeradbiomed.2015.08.003. Epub 2015 Sep 21. Free Radic Biol Med. 2015. PMID: 26393422 Free PMC article. Review.
Cited by
-
Oxidation Products of Tryptophan and Proline in Adipokinetic Hormones-Artifacts or Post-Translational Modifications?Life (Basel). 2023 Dec 10;13(12):2315. doi: 10.3390/life13122315. Life (Basel). 2023. PMID: 38137917 Free PMC article.
-
Toxic Cyanobacteria in Svalbard: Chemical Diversity of Microcystins Detected Using a Liquid Chromatography Mass Spectrometry Precursor Ion Screening Method.Toxins (Basel). 2018 Apr 3;10(4):147. doi: 10.3390/toxins10040147. Toxins (Basel). 2018. PMID: 29614044 Free PMC article.
-
Further characterization of glycine-containing microcystins from the McMurdo dry Valleys of Antarctica.Toxins (Basel). 2015 Feb 10;7(2):493-515. doi: 10.3390/toxins7020493. Toxins (Basel). 2015. PMID: 25675414 Free PMC article.
-
Chemical diversity of cyanobacterial natural products.Nat Prod Rep. 2025 Jan 22;42(1):6-49. doi: 10.1039/d4np00040d. Nat Prod Rep. 2025. PMID: 39540765 Review.
-
Moving towards adaptive management of cyanotoxin-impaired water bodies.Microb Biotechnol. 2016 Sep;9(5):641-51. doi: 10.1111/1751-7915.12383. Epub 2016 Jul 15. Microb Biotechnol. 2016. PMID: 27418325 Free PMC article. Review.
References
-
- Rinehart K., Namikoshi M., Choi B. Structure and biosynthesis of toxins from blue-green algae (cyanobacteria) J. Appl. Phycol. 1994;6:159–176. doi: 10.1007/BF02186070. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

