An aeroplysinin-1 specific nitrile hydratase isolated from the marine sponge Aplysina cavernicola
- PMID: 23966036
- PMCID: PMC3766881
- DOI: 10.3390/md11083046
An aeroplysinin-1 specific nitrile hydratase isolated from the marine sponge Aplysina cavernicola
Abstract
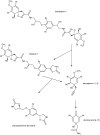
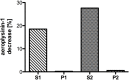
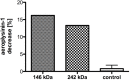
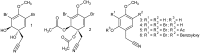
A nitrile hydratase (NHase) that specifically accepts the nitrile aeroplysinin-1 (1) as a substrate and converts it into the dienone amide verongiaquinol (7) was isolated, partially purified and characterized from the Mediterranean sponge Aplysina cavernicola; although it is currently not known whether the enzyme is of sponge origin or produced by its symbiotic microorganisms. The formation of aeroplysinin-1 and of the corresponding dienone amide is part of the chemical defence system of A. cavernicola. The latter two compounds that show strong antibiotic activity originate from brominated isoxazoline alkaloids that are thought to protect the sponges from invasion of bacterial pathogens. The sponge was shown to contain at least two NHases as two excised protein bands from a non denaturating Blue Native gel showed nitrile hydratase activity, which was not observed for control samples. The enzymes were shown to be manganese dependent, although cobalt and nickel ions were also able to recover the activity of the nitrile hydratases. The temperature and pH optimum of the studied enzymes were found at 41 °C and pH 7.8. The enzymes showed high substrate specificity towards the physiological substrate aeroplysinin-1 (1) since none of the substrate analogues that were prepared either by partial or by total synthesis were converted in an in vitro assay. Moreover de-novo sequencing by mass spectrometry was employed to obtain information about the primary structure of the studied NHases, which did not reveal any homology to known NHases.
Figures




Similar articles
-
Chemical defense of Mediterranean sponges Aplysina cavernicola and Aplysina aerophoba.Z Naturforsch C J Biosci. 2004 Jan-Feb;59(1-2):113-22. doi: 10.1515/znc-2004-1-222. Z Naturforsch C J Biosci. 2004. PMID: 15018063
-
Activated chemical defense in aplysina sponges revisited.J Chem Ecol. 2006 Jan;32(1):97-123. doi: 10.1007/s10886-006-9355-x. Epub 2006 Feb 26. J Chem Ecol. 2006. PMID: 16525873
-
Probing the enantioselectivity of a diverse group of purified cobalt-centred nitrile hydratases.Org Biomol Chem. 2011 Apr 21;9(8):3011-9. doi: 10.1039/c0ob01067g. Epub 2011 Mar 7. Org Biomol Chem. 2011. PMID: 21380438
-
Sponge derived bromotyrosines: structural diversity through natural combinatorial chemistry.Nat Prod Commun. 2015 Jan;10(1):219-31. Nat Prod Commun. 2015. PMID: 25920247 Review.
-
Aeroplysinin-1, a Sponge-Derived Multi-Targeted Bioactive Marine Drug.Mar Drugs. 2015 Dec 22;14(1):1. doi: 10.3390/md14010001. Mar Drugs. 2015. PMID: 26703630 Free PMC article. Review.
Cited by
-
Applications of Marine-Derived Microorganisms and Their Enzymes in Biocatalysis and Biotransformation, the Underexplored Potentials.Front Microbiol. 2019 Aug 20;10:1453. doi: 10.3389/fmicb.2019.01453. eCollection 2019. Front Microbiol. 2019. PMID: 31481935 Free PMC article. Review.
-
Co-occurring nitrifying symbiont lineages are vertically inherited and widespread in marine sponges.ISME J. 2024 Jan 8;18(1):wrae069. doi: 10.1093/ismejo/wrae069. ISME J. 2024. PMID: 38676557 Free PMC article.
-
Cultivation of Bacteria From Aplysina aerophoba: Effects of Oxygen and Nutrient Gradients.Front Microbiol. 2020 Feb 19;11:175. doi: 10.3389/fmicb.2020.00175. eCollection 2020. Front Microbiol. 2020. PMID: 32140143 Free PMC article.
-
Diving into the Molecular Diversity of Aplysina cavernicola's Exometabolites: Contribution of Bromo-Spiroisoxazoline Alkaloids.ACS Omega. 2022 Nov 16;7(47):43068-43083. doi: 10.1021/acsomega.2c05415. eCollection 2022 Nov 29. ACS Omega. 2022. PMID: 36467926 Free PMC article.
-
Secondary Metabolome Variability and Inducible Chemical Defenses in the Mediterranean Sponge Aplysina cavernicola.J Chem Ecol. 2016 Jan;42(1):60-70. doi: 10.1007/s10886-015-0664-9. Epub 2016 Jan 12. J Chem Ecol. 2016. PMID: 26757731
References
-
- Putz A., Proksch P. Chemical defence in marine ecosystems. Annu. Plant Rev. 2010;39:162–213.
-
- Paul V.J., van Alstyne K.L. Activation of chemical defenses in the tropical green algae Halimeda spp. J. Exp. Mar. Biol. Ecol. 1992;160:191–203. doi: 10.1016/0022-0981(92)90237-5. - DOI
-
- Jung V., Pohnert G. Rapid wound-activated transformation of the green algal defensive metabolite caulerpenyne. Tetrahedron. 2001;57:7169–7172. doi: 10.1016/S0040-4020(01)00692-5. - DOI
-
- Teeyapant R., Proksch P. Biotransformation of brominated compounds in the marine sponge Verongia aerophoba—evidence for an induced chemical defense? Naturwissenschaften. 1993;80:369–370. doi: 10.1007/BF01138794. - DOI
-
- Ciminiello P., Dell’Aversano C., Fattorusso E., Magno S., Carrano L., Pansini M. Chemistry of Verongida sponges. VII. Bromo compounds from the Caribbean sponge Aplysina archeri. Tetrahedron. 1996;52:9863–9868. doi: 10.1016/0040-4020(96)00518-2. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

