The click reaction as an efficient tool for the construction of macrocyclic structures
- PMID: 23966075
- PMCID: PMC6270095
- DOI: 10.3390/molecules18089512
The click reaction as an efficient tool for the construction of macrocyclic structures
Abstract
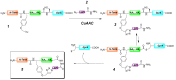
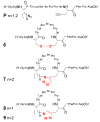
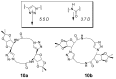
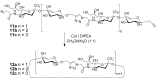
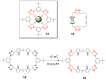
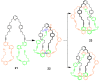
The Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC, known as the click reaction) is an established tool used for the construction of complex molecular architectures. Given its efficiency it has been widely applied for bioconjugation, polymer and dendrimer synthesis. More recently, this reaction has been utilized for the efficient formation of rigid or shape-persistent, preorganized macrocyclic species. This strategy also allows the installment of useful functionalities, in the form of polar and function-rich 1,2,3-triazole moieties, directly embedded in the macrocyclic structures. This review analyzes the state of the art in this context, and provides some elements of perspective for future applications.
Figures



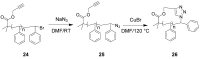
Similar articles
-
Synthesis of macrocyclic peptidomimetics via the Ugi-click-strategy.Org Biomol Chem. 2019 Mar 27;17(13):3433-3445. doi: 10.1039/c9ob00229d. Org Biomol Chem. 2019. PMID: 30874270
-
Efficient access to new chemical space through flow--construction of druglike macrocycles through copper-surface-catalyzed azide-alkyne cycloaddition reactions.Chemistry. 2010 Dec 27;16(48):14506-12. doi: 10.1002/chem.201002215. Chemistry. 2010. PMID: 21038332
-
Synthesis of novel tryptamine-based macrocycles using an Ugi 4-CR/microwave assisted click-cycloaddition reaction protocol.Org Biomol Chem. 2015 Apr 21;13(15):4408-12. doi: 10.1039/c5ob00067j. Org Biomol Chem. 2015. PMID: 25766574
-
CuAAC: An Efficient Click Chemistry Reaction on Solid Phase.ACS Comb Sci. 2016 Jan 11;18(1):1-14. doi: 10.1021/acscombsci.5b00087. Epub 2015 Dec 21. ACS Comb Sci. 2016. PMID: 26652044 Review.
-
Discovery of novel anti-HIV agents via Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) click chemistry-based approach.Expert Opin Drug Discov. 2016 Sep;11(9):857-71. doi: 10.1080/17460441.2016.1210125. Epub 2016 Jul 21. Expert Opin Drug Discov. 2016. PMID: 27400283 Review.
Cited by
-
Aza-Oxa-Triazole Based Macrocycles with Tunable Properties: Design, Synthesis, and Bioactivity.Molecules. 2022 May 25;27(11):3409. doi: 10.3390/molecules27113409. Molecules. 2022. PMID: 35684347 Free PMC article.
-
Red light-emitting short Mango-based system enables tracking a mycobacterial small noncoding RNA in infected macrophages.Nucleic Acids Res. 2023 Apr 11;51(6):2586-2601. doi: 10.1093/nar/gkad100. Nucleic Acids Res. 2023. PMID: 36840712 Free PMC article.
-
Nucleoside macrocycles formed by intramolecular click reaction: efficient cyclization of pyrimidine nucleosides decorated with 5'-azido residues and 5-octadiynyl side chains.Beilstein J Org Chem. 2018 Sep 13;14:2404-2410. doi: 10.3762/bjoc.14.217. eCollection 2018. Beilstein J Org Chem. 2018. PMID: 30254706 Free PMC article.
-
Cyclic polymers from alkynes.Nat Chem. 2016 Aug;8(8):791-6. doi: 10.1038/nchem.2516. Epub 2016 May 16. Nat Chem. 2016. PMID: 27442285
-
The intramolecular click reaction using 'carbocontiguous' precursors.Tetrahedron. 2017 Jul 20;73(29):4206-4213. doi: 10.1016/j.tet.2016.11.016. Epub 2016 Nov 5. Tetrahedron. 2017. PMID: 28943665 Free PMC article.
References
-
- IUPAC GOLDBOOK. [(accessed on 18 June 2013)]. Available online: http://goldbook.iupac.org/M03662.html.
-
- Lehn J.M. Supramolecular chemistry—scope and perspectives molecules, supermolecules, and molecular devices (nobel lecture) Angew. Chem. Int. Ed. 1988;27:89–112. doi: 10.1002/anie.198800891. - DOI
-
- Pedersen C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967;89:7017–7036. doi: 10.1021/ja01002a035. - DOI
-
- Weber E., Vögtle F., Burrell A. K. Macrocycles. Springer-Verlag; Berkin, Germany: 1992.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

