Enantio- and periselective nitroalkene Diels-Alder reactions catalyzed by helical-chiral hydrogen bond donor catalysts
- PMID: 23966083
- PMCID: PMC6270221
- DOI: 10.3390/molecules18089982
Enantio- and periselective nitroalkene Diels-Alder reactions catalyzed by helical-chiral hydrogen bond donor catalysts
Abstract
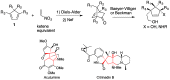
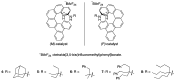
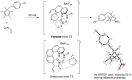
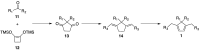
Helical-chiral double hydrogen bond donor catalysts promote the nitroalkene Diels-Alder reaction in an enantio- and periselective manner. This represents the first asymmetric catalytic nitroalkene Diels-Alder reaction via LUMO-lowering catalysis. To gain an insight into this new process, the substrate scope of our catalyst was investigated by exploiting readily available 5-substituted pentamethylcyclopentadienes. The catalyst was found to tolerate dienes with different steric demands as well as dienes substituted with heteroatoms. The synthetic utility of 5-substituted pentamethylcyclopentadienes is rather limited, and thus we have developed a three-step route to 1,4,5,5-tetrasubstituted cyclopentadienes from commercially available ketones.
Figures
Similar articles
-
Enantio- and periselective nitroalkene Diels-Alder reaction.Org Biomol Chem. 2012 Dec 14;10(46):9134-6. doi: 10.1039/c2ob26674a. Epub 2012 Oct 29. Org Biomol Chem. 2012. PMID: 23104427
-
Magnesium(II)-catalyzed asymmetric hetero-Diels-Alder reaction of Brassard's dienes with isatins.Chem Commun (Camb). 2014 Jan 28;50(8):994-6. doi: 10.1039/c3cc47800a. Chem Commun (Camb). 2014. PMID: 24305614
-
Enantioselective access to tricyclic tetrahydropyran derivatives by a remote hydrogen bonding mediated intramolecular IEDHDA reaction.Nat Commun. 2021 Dec 10;12(1):7188. doi: 10.1038/s41467-021-27521-z. Nat Commun. 2021. PMID: 34893616 Free PMC article.
-
Boron and Silicon-Substituted 1,3-Dienes and Dienophiles and Their Use in Diels-Alder Reactions.Molecules. 2020 Aug 16;25(16):3740. doi: 10.3390/molecules25163740. Molecules. 2020. PMID: 32824327 Free PMC article. Review.
-
[Catalytic Synthesis of Optically Active Polycyclic Heterocyclic Compounds].Yakugaku Zasshi. 2020;140(10):1213-1224. doi: 10.1248/yakushi.20-00137. Yakugaku Zasshi. 2020. PMID: 32999200 Review. Japanese.
Cited by
-
The inverted ketene synthon: a double umpolung approach to enantioselective β2,3-amino amide synthesis.Chem Sci. 2018 Nov 12;10(4):1138-1143. doi: 10.1039/c8sc04330b. eCollection 2019 Jan 28. Chem Sci. 2018. PMID: 30774911 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources