Total synthesis, cytotoxic effects of damnacanthal, nordamnacanthal and related anthraquinone analogues
- PMID: 23966087
- PMCID: PMC6269871
- DOI: 10.3390/molecules180810042
Total synthesis, cytotoxic effects of damnacanthal, nordamnacanthal and related anthraquinone analogues
Abstract
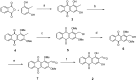
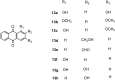
Naturally occurring anthraquinones, damnacanthal (1) and nordamnacanthal (2) were synthesized with modified reaction steps and investigated for their cytotoxicity against the MCF-7 and K-562 cancer cell lines, respectively. Intermediate analogues 2-bromomethyl-1,3-dimethoxyanthraquinone (5, IC50 = 5.70 ± 0.21 and 8.50 ± 1.18 mg/mL), 2-hydroxymethyl-1,3-dimethoxyanthraquinone (6, IC50 = 12.10 ± 0.14 and 14.00 ± 2.13), 2-formyl-1,3-dimethoxyantharquinone (7, IC50 = 13.10 ± 1.02 and 14.80 ± 0.74), 1,3-dimethoxy-2-methylanthraquinone (4, IC50 = 9.40 ± 3.51 and 28.40 ± 2.33), and 1,3-dihydroxy-2-methylanthraquinone (3, IC50 = 25.60 ± 0.42 and 28.40 ± 0.79) also exhibited moderate cytotoxicity against MCF-7 and K-562 cancer cell lines, respectively. Other structurally related compounds like 1,3-dihydroxyanthraquinone (13a, IC50 = 19.70 ± 0.35 and 14.50 ± 1.28), 1,3-dimethoxyanthraquinone (13b, IC50 = 6.50 ± 0.66 and 5.90 ± 0.95) were also showed good cytotoxicity. The target compound damnacanthal (1) was found to be the most cytotoxic against the MCF-7 and K-562 cancer cell lines, with IC50 values of 3.80 ± 0.57 and 5.50 ± 1.26, respectively. The structures of all compounds were elucidated with the help of detailed spectroscopic techniques.
Figures
Similar articles
-
Anticancer Potential of Damnacanthal and Nordamnacanthal from Morinda elliptica Roots on T-lymphoblastic Leukemia Cells.Molecules. 2021 Mar 12;26(6):1554. doi: 10.3390/molecules26061554. Molecules. 2021. PMID: 33808969 Free PMC article.
-
Anthraquinones from the roots of Prismatomeris malayana.Nat Prod Res. 2008;22(11):962-8. doi: 10.1080/14786410701650261. Nat Prod Res. 2008. PMID: 18629711
-
A new anthraquinone from Morinda citrifolia roots.Nat Prod Res. 2009;23(14):1322-9. doi: 10.1080/14786410902753138. Nat Prod Res. 2009. PMID: 19735047
-
Synthesis of an anthraquinone derivative (DHAQC) and its effect on induction of G2/M arrest and apoptosis in breast cancer MCF-7 cell line.Drug Des Devel Ther. 2015 Feb 17;9:983-92. doi: 10.2147/DDDT.S65468. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25733816 Free PMC article.
-
Damnacanthal: a promising compound as a medicinal anthraquinone.Anticancer Agents Med Chem. 2014 Jun;14(5):750-5. doi: 10.2174/18715206113136660366. Anticancer Agents Med Chem. 2014. PMID: 24164045 Review.
Cited by
-
Novel Zinc(II) Complexes [Zn(atc-Et)₂] and [Zn(atc-Ph)₂]: In Vitro and in Vivo Antiproliferative Studies.Int J Mol Sci. 2016 May 21;17(5):781. doi: 10.3390/ijms17050781. Int J Mol Sci. 2016. PMID: 27213368 Free PMC article.
-
Anticancer Potential of Damnacanthal and Nordamnacanthal from Morinda elliptica Roots on T-lymphoblastic Leukemia Cells.Molecules. 2021 Mar 12;26(6):1554. doi: 10.3390/molecules26061554. Molecules. 2021. PMID: 33808969 Free PMC article.
-
Targeted tumor therapy by Rubia tinctorum L.: analytical characterization of hydroxyanthraquinones and investigation of their selective cytotoxic, adhesion and migration modulator effects on melanoma cell lines (A2058 and HT168-M1).Cancer Cell Int. 2015 Dec 18;15:119. doi: 10.1186/s12935-015-0271-4. eCollection 2015. Cancer Cell Int. 2015. PMID: 26690297 Free PMC article.
-
Microbial Synthesis of Non-Natural Anthraquinone Glucosides Displaying Superior Antiproliferative Properties.Molecules. 2018 Aug 28;23(9):2171. doi: 10.3390/molecules23092171. Molecules. 2018. PMID: 30154376 Free PMC article.
-
Journey of anthraquinones as anticancer agents - a systematic review of recent literature.RSC Adv. 2021 Nov 5;11(57):35806-35827. doi: 10.1039/d1ra05686g. eCollection 2021 Nov 4. RSC Adv. 2021. PMID: 35492773 Free PMC article. Review.
References
-
- Murphy G.P., Lawrence W.J., Lenhard R.E. American Society Textbook of Clinical Oncology. 2nd ed. American Cancer Society; Atlanta, GA, USA: 1995.
-
- Doroshow J.H. Anthracyclines and anthracenediones. In: Chabner B.A., Longo D.L., editors. Cancer Chemotherapy and Biotherapy: Principles and Practice. 2nd ed. Lippincott-Raven Publishers; Philadelphia, PA, USA: 1996. pp. 409–434.
-
- Faulds D., Balfour J.A., Chrisp P., Langtry H.D. Mitoxantrone, a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in the chemotherapy of cancer. Drugs. 1991;3:400–449. - PubMed
-
- Kosalec I., Kremer D., Locatelli M., Epifano F., Genovese S., Carlucci G., Randic M., Koncic Z.M. Anthraquinone profile, antioxidant and antimicrobial activity of bark extracts of Rhamnus alaternus, R. fallax, R. intermedia and R. pumila. Food Chem. 2013;136:335–341. doi: 10.1016/j.foodchem.2012.08.026. - DOI - PubMed
-
- Shi Y.Q., Fukai T., Sakagami H., Kuroda J., Miyaoka R., Tamura M., Yoshida N., Nomura T. Cytotoxic and DNA damage-inducing activities of low molecular weight phenols from rhubarb. Anticancer Res. 2001;21:2847–2853. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources