Combined inhaled anticholinergics and short-acting beta2-agonists for initial treatment of acute asthma in children
- PMID: 23966133
- PMCID: PMC12047668
- DOI: 10.1002/14651858.CD000060.pub2
Combined inhaled anticholinergics and short-acting beta2-agonists for initial treatment of acute asthma in children
Abstract
Background: There are several treatment options for managing acute asthma exacerbations (sustained worsening of symptoms that do not subside with regular treatment and require a change in management). Guidelines advocate the use of inhaled short acting beta2-agonists (SABAs) in children experiencing an asthma exacerbation. Anticholinergic agents, such as ipratropium bromide and atropine sulfate, have a slower onset of action and weaker bronchodilating effect, but may specifically relieve cholinergic bronchomotor tone and decrease mucosal edema and secretions. Therefore, the combination of inhaled anticholinergics with SABAs may yield enhanced and prolonged bronchodilation.
Objectives: To determine whether the addition of inhaled anticholinergics to SABAs provides clinical improvement and affects the incidence of adverse effects in children with acute asthma exacerbations.
Search methods: We searched MEDLINE (1966 to April 2000), EMBASE (1980 to April 2000), CINAHL (1982 to April 2000) and reference lists of studies of previous versions of this review. We also contacted drug manufacturers and trialists. For the 2012 review update, we undertook an 'all years' search of the Cochrane Airways Group's register on the 18 April 2012.
Selection criteria: Randomized parallel trials comparing the combination of inhaled anticholinergics and SABAs with SABAs alone in children (aged 18 months to 18 years) with an acute asthma exacerbation.
Data collection and analysis: Two review authors independently assessed trial quality and extracted data. We used the GRADE rating system to assess the quality of evidence for our primary outcome (hospital admission).
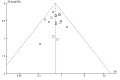
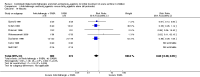
Main results: Twenty trials met the review eligibility criteria, generated 24 study comparisons and comprised 2697 randomised children aged one to 18 years, presenting predominantly with moderate or severe exacerbations. Most studies involved both preschool-aged children and school-aged children; three studies also included a small proportion of infants less than 18 months of age. Nine trials (45%) were at a low risk of bias. Most trials used a fixed-dose protocol of three doses of 250 mcg or two doses of 500 mcg of nebulized ipratropium bromide in combination with a SABA over 30 to 90 minutes while three trials used a single dose and two used a flexible-dose protocol according to the need for SABA.The addition of an anticholinergic to a SABA significantly reduced the risk of hospital admission (risk ratio (RR) 0.73; 95% confidence interval (CI) 0.63 to 0.85; 15 studies, 2497 children, high-quality evidence). In the group receiving only SABAs, 23 out of 100 children with acute asthma were admitted to hospital compared with 17 (95% CI 15 to 20) out of 100 children treated with SABAs plus anticholinergics. This represents an overall number needed to treat for an additional beneficial outcome (NNTB) of 16 (95% CI 12 to 29).Trends towards a greater effect with increased treatment intensity and with increased asthma severity were observed, but did not reach statistical significance. There was no effect modification due to concomitant use of oral corticosteroids and the effect of age could not be explored. However, exclusion of the one trial that included infants (< 18 months) and contributed data to the main outcome, did not affect the results. Statistically significant group differences favoring anticholinergic use were observed for lung function, clinical score at 120 minutes, oxygen saturation at 60 minutes, and the need for repeat use of bronchodilators prior to discharge from the emergency department. No significant group difference was seen in relapse rates.Fewer children treated with anticholinergics plus SABA reported nausea and tremor compared with SABA alone; no significant group difference was observed for vomiting.
Authors' conclusions: Children with an asthma exacerbation experience a lower risk of admission to hospital if they are treated with the combination of inhaled SABAs plus anticholinergic versus SABA alone. They also experience a greater improvement in lung function and less risk of nausea and tremor. Within this group, the findings suggested, but did not prove, the possibility of an effect modification, where intensity of anticholinergic treatment and asthma severity, could be associated with greater benefit.Further research is required to identify the characteristics of children that may benefit from anticholinergic use (e.g. age and asthma severity including mild exacerbation and impending respiratory failure) and the treatment modalities (dose, intensity, and duration) associated with most benefit from anticholinergic use better.
Conflict of interest statement
Francine Ducharme has received travel support, research funds and fees for speaking from Novartis, Taketa (formally Nycomed) and Merck Frosst Inc and served on an advisory board for Novartis.
Figures






















Update of
-
Combined inhaled anticholinergics and beta2-agonists for initial treatment of acute asthma in children.Cochrane Database Syst Rev. 2000;(4):CD000060. doi: 10.1002/14651858.CD000060. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2013 Aug 21;(8):CD000060. doi: 10.1002/14651858.CD000060.pub2. PMID: 11034671 Updated.
Comment in
-
Combined anti-cholinergic and short-acting β-agonist therapy reduces hospital admissions for acute asthma.J Paediatr Child Health. 2014 Jul;50(7):577-8. doi: 10.1111/jpc.12669. J Paediatr Child Health. 2014. PMID: 25041304 No abstract available.
References
References to studies included in this review
Beck 1985 {published data only}
-
- Beck R. Use of ipratropium bromide by inhalation in the treatment of acute asthma in children. Clinical experience [in French] [Utilisation du bromure d'ipratropium par voie inhalée pour le traitement de l'asthme aigu chez l'enfant]. Archives de Pediatrie 1995;2 Suppl(2):145‐8. - PubMed
-
- Beck R, Robertson C, Galdes‐Sebaldt M, Levison H. Combined salbutamol and ipratropium bromide by inhalation in the treatment of severe acute asthma. Journal of Pediatrics 1985;107:605‐8. - PubMed
Benito Fernandez 2000 {published data only}
-
- Benito Fernandez J, Mintegui Raso S, Sanchez Echaniz J, Vazquez Ronco MA, Pijoan Zubizarreta JI. Efficacy of early administration of nebulized ipratropium bromide in children with asthmatic crisis [in Spanish]. Anales Espanoles de Pediatria 2000;53(3):217‐22. - PubMed
BI [pers comm] {published data only}
-
- Boehringer Ingelheim Pharmaceuticals. A comparison of Combivent UDV (ipratropium 500mcg and salbutamol 2.5mg) and salbutamol UDV alone (2.5mg). Personal communication from Boehringer Ingelheim 2009.
Calvo 1998 {published data only}
-
- Calvo GM, Calvo AM, Marin HF, Moya GJ. Is it useful to add an anticholinergic treatment to beta2‐adrenergic medication in acute asthma attack. Journal of Investigative Allergology Clinical Immunology 1998;8(1):30‐4. - PubMed
Chakraborti 2006 {published data only}
-
- Chakraborti A, Lodha R, Pandey RM, Kabra SK. Randomized controlled trial of ipratropium bromide and salbutamol versus salbutamol alone in children with acute exacerbation of asthma. Indian Journal of Pediatrics. 2006;73(11):979‐83. - PubMed
Cook 1985 {published data only}
-
- Cook JJ, Fergusson DM, Dawson KP. Ipratropium and fenoterol in the treatment of acute asthma. Pharmatherapeutica 1985;4(6):383‐6. - PubMed
Ducharme 1998 {unpublished data only}
-
- Ducharme FM, Davis GM. Randomized controlled trial of ipratropium bromide and frequent low doses of salbutamol in the treatment of mild and moderate acute asthma. Journal of Pediatrics 1998;133(4):479‐85. - PubMed
Guill 1987 {published data only}
-
- Guill MF, Maloney MJ, DuRant RH. Comparison of inhaled metaproterenol, inhaled atropine sulfate and their combination in treatment of children with acute asthma. Annals of Allergy 1987;59:367‐71. - PubMed
Iramain 2011 {published data only}
-
- Iramain R, Lopez‐Herce J, Coronel J, Spitters C, Guggiari J, Bogado N. inhaled salbutamol plus ipratropium in moderate and severe asthma crises in children. Journal of Asthma 2011;48:298‐303. - PubMed
Peterson 1996 {unpublished data only}
-
- Peterson R, Wensley D, Mitchell I, Klassen T, Lamarre J, Rivard G, et al. Boehringer Ingelheim Trial No 2442430.3. Boehringer Ingelheim 1994.
Phanichyakam 1990 {published data only}
-
- Phanichyakam P, Kraisarin C, Sasisakulporn C. Comparison of inhaled terbutaline and inhaled terbutaline plus ipratropium bromide in acute asthmatic children. Asian Pacific Journal of Allergy and Immunology 1990;8:45‐8. - PubMed
Qureshi 1997 {published data only}
-
- Qureshi FA, Zaritsky A, Lakkis H. Efficacy of nebulized ipratropium in severe asthmatic children. Annals of Emergency Medicine 1997;29:205‐11. - PubMed
Qureshi 1998 {published data only}
-
- Qureshi F, Pestian J, Davis P, Zaritsky A. Effect of nebulized ipratropium on the hospitalization rates of children with asthma. New England Journal of Medicine 1998;339(15):1030‐5. - PubMed
Qureshi 1998 (moderate) {published data only}
-
- Qureshi F, Pestian J, Davis P, Zaritsky A. Effect of nebulized ipratropium on the hospitalization rates of children with asthma. New England Journal of Medicine 1998;339(15):1030‐5. - PubMed
Qureshi 1998 (severe) {published data only}
-
- Qureshi F, Pestian J, Davis P, Zaritsky A. Effect of nebulized ipratropium on the hospitalization rates of children with asthma. New England Journal of Medicine 1998;339(15):1030‐35. - PubMed
Reisman 1988 {published data only}
-
- Reisman J, Galdes‐Sebaldt M, Kazim F, Canny G, Levison H. Frequent administration by inhalation of salbutamol and ipratropium bromide in the initial management of severe acute asthma in children. Journal of Allergy & Clinical Immunology 1988;81:16‐20. - PubMed
Schuh 1995 {published data only}
-
- Schuh S, Johnson DW, Callahan S, Canny G, Levison H. Efficacy of frequent nebulized ipratropium added to frequent high‐dose albuterol therapy in severe childhood asthma. Journal of Pediatrics 1995;126:639‐45. - PubMed
Schuh 1995 (multiple) {published data only}
-
- Schuh S, Johnson DW, Callahan S, Canny G, Levison H. Efficacy of frequent nebulized ipratropium added to frequent high‐dose albuterol therapy in severe childhood asthma. Journal of Pediatrics 1995;126:639‐45. - PubMed
Schuh 1995 (single) {published data only}
-
- Schuh S, Johnson DW, Callahan S, Canny G, Levison H. Efficacy of frequent nebulized ipratropium added to frequent high‐dose albuterol therapy in severe childhood asthma. Journal of Pediatrics 1995;126:639‐45. - PubMed
Sharma 2004 {published data only}
-
- Sharma A, Madaan A. Nebulized salbutamol vs salbutamol and ipratropium combination in asthma. Indian Journal of Pediatrics 2004;71(2):121‐4. - PubMed
Sienra Monge 2000 {published data only}
-
- Sienra Monge JJ, Bermejo Guevara MA, Rio Navarro BE, Rosas Vargas MA, Reyes Ruiz NI. Degree and duration of bronchodilatation with an agonist beta 2 administered alone versus an agonist beta 2 administered with ipratropium bromide in children with acute asthma. Revista Alergia Mexico 2000;47(1):26‐9. - PubMed
Watanasomsiri 2006 {published data only}
-
- Watanasomsiri A, Phipatanakul W. Comparison of nebulized ipratropium bromide with salbutamol versus salbutamol alone in acute asthma exacerbation in children [Abstract]. Chest 2004;126(4 Suppl):761S‐b. - PubMed
-
- Watanasomsiri A, Phipatanakul W. Comparison of nebulized ipratropium bromide with salbutamol vs salbutamol alone in acute asthma exacerbation in children. Annals of Allergy, Asthma, & Immunology 2006;96(5):701‐6. - PubMed
Watson 1988 {published data only}
-
- Watson WTA, Becker AB, Simmons FER. Comparison of ipratropium solution, fenoterol solution and their combination administered by nebulizer and face mask to children with acute asthma. Journal of Allergy & Clinical Immunology 1988;82:1012‐8. - PubMed
Zorc 1999 {published data only}
-
- Zorc JJ, Pusic MV, Ogborn CJ, Lebet R, Duggan AK. Ipratropium bromide added to asthma treatment in the pediatric emergency department. Pediatrics 1999;103(4):748‐52. - PubMed
Zorc 1999 (mild) {published data only}
-
- Zorc JJ, Pusic MV, Ogborn CJ, Lebet R, Duggan AK. Ipratropium bromide added to asthma treatment in the pediatric emergency department. Pediatrics 1999;103(4):748‐52. - PubMed
Zorc 1999 (moderate) {published data only}
-
- Zorc JJ, Pusic MV, Ogborn CJ, Lebet R, Duggan AK. Ipratropium bromide added to asthma treatment in the pediatric emergency department. Pediatrics 1999;103(4):748‐52. - PubMed
Zorc 1999 (severe) {published data only}
-
- Zorc JJ, Pusic, MV, Ogborn CJ, Lebet R, Duggan AK. Ipratropium bromide added to asthma treatment in the pediatric emergency department. Pediatrics 1999;103(4):748‐52. - PubMed
References to studies excluded from this review
Anthracopoulos 2005 {published data only}
-
- Anthracopoulos M, Karatza A, Davlouros PA, Beratis NG. Comparison of salbutamol (SAL) and combined salbutamol and ipratropium bromide (SAL+IB) nebulization therapy in acute asthma exacerbations (AAE) in paediatric patients. European Respiratory Journal 2000;16 Suppl 31:304s.
-
- Anthracopoulos MB, Karatza AA, Davlouros PA, Chiladakis JA, Manolis AS, Beratis NG. Effects of two nebulization regimens on heart rate variability during acute asthma exacerbations in children. Journal of Asthma 2005;42(4):273‐9. - PubMed
Boner 1987 {published data only}
-
- Boner AL, Stefano G, Niero E, Vallone G, Gaburro D. Salbutamol and ipratropium bromide solution in the treatment of bronchospasm in asthmatic children. Annals of Allergy 1987;58:54‐8. - PubMed
Bratteby 1986 {published data only}
-
- Bratteby L‐E, Foucard T, Lonnerholm G. Combined treatment with ipratropium bromide and beta‐2‐adrenoceptor agonists in childhood asthma. American Review of Respiratory Disease 1986;68:239‐47. - PubMed
Browne 2002 {published data only}
-
- Browne GJ, Trieu L, Asperen P. Randomized, double‐blind, placebo‐controlled trial of intravenous salbutamol and nebulized ipratropium bromide in early management of severe acute asthma in children presenting to an emergency department. Critical Care Medicine 2002;30(2):448‐53. - PubMed
Caubet 1989 {published data only}
-
- Caubet Y. Comparison of the effectiveness and the tolerance of the dosing aerosols of fenoterol/ipratropium against salbutamol in the asthmatic child [in French] [Comparaison de l'efficacite et de la tolerance des aerosols doseurs de l'association fenoterol/ipratropium contre le salbutamol chez le grand enfant asthmatique]. La Revue de Pediatrie 1989;25(2):74‐6.
Craven 2001 {published data only}
-
- Craven D, Kercsmar CM, Myers TR, O'Riordan MA, Golonka G, Moore S. Ipratropium bromide plus nebulized albuterol for the treatment of hospitalized children with acute asthma. Journal of Pediatrics 2001;138(1):51‐8. - PubMed
Davis 1984 {published data only}
-
- Davis A, Vickerson F, Worsley G, Mindorff C, Kazim F, Levison H. Determination of dose‐response relationship for nebulized ipratropium in asthmatic children. Journal of Pediatrics 1984;Dec:1002‐5. - PubMed
Delacourt 1994 {published data only}
-
- Delacourt C, Blic J, Lebourgeois M, Scheinmann P. Value of ipratropium bromide in asthma crisis in children [in French] [Interet du bromure d'ipratropium dans la crise d'asthme de l'enfant]. Archives de Pediatrie 1994;1:87‐92. - PubMed
DeStefano 1989 {published data only}
-
- DeStefano G, Bonetti S, Bonizzato C, Valletta EA, Piacentini GL, Boner AL. Additive effect of albuterol and ipratropium bromide in the treatment of bronchospasm in children. Annals of Allergy 1989;65(4):260‐2. - PubMed
Ekwo 1978 {published data only}
-
- Ekwo E, Weinberger M. Evaluation of a program for the pharmacologic management of children with asthma. Journal of Allergy & Clinical Immunology 1978;61(4):240‐7. - PubMed
Freeman 1989 {published data only}
-
- Freeman J, Landau LI. The effects of ipratropium bromide and fenoterol nebulizer solutions in children with asthma. Clinical Pediatrics 1989;28(12):556‐60. - PubMed
Friberg 1989 {published data only}
-
- Friberg S, Graff‐Lonnevig V. Ipratropium bromide (Atrovent) in childhood asthma: a cumulative dose‐response study. Annals of Allergy 1989;62:131‐4. - PubMed
Goggin 2001 {published data only}
-
- Goggin N, Macarthur C, Parkin PC. Randomized trial of the addition of ipratropium bromide to albuterol and corticosteroid therapy in children hospitalized because of an acute asthma exacerbation. Archives of Pediatrics & Adolescent Medicine 2001;155(12):1329‐34. - PubMed
Greenough 1986 {published data only}
-
- Greenough A, Yuksel B, Everett L, Price JF. Inhaled ipratropium bromide and terbutaline in asthmatic children. Respiratory Medicine 1993;87:111‐4. - PubMed
Groggins 1981 {published data only}
Hodges 1981 {published data only}
Kumaratne 2003 {published data only}
-
- Kumaratne M, Gunawardane G. Addition of ipratropium to nebulized albuterol in children with acute asthma presenting to a pediatric office. Clinical Pediatrics 2003;42(2):127‐32. - PubMed
Lenney 1986 {published data only}
-
- Lenney W, Evans NAP. Nebulized salbutamol and ipratropium bromide in asthmatic children. British Journal of Diseases of the Chest 1986;80:59‐65. - PubMed
Mann 1982 {published data only}
O'Driscoll 1989 {published data only}
-
- O'Driscoll BR, Taylor RJ, Horsley MG, Chambers DK, Bernstein A. Nebulised salbutamol with and without ipratropium bromide in acute airflow obstruction. Lancet 1989;1(8652):1418‐20. - PubMed
Paredes 1994 {published data only}
-
- Paredes N, Sol M, Rio N, Sienra M. Comparative efficacy of salbutamol versus salbutamol with ipratropium bromide in acute asthma in children [Abstract]. XV International Congress of Allergology and Clinical Immunology: Annual Meeting of the European Academy of Allergology and Clinical Immunology. 1994:377.
Ralston 2005 {published data only}
-
- Ralston ME, Euwema MS, Knecht KR, Ziolkowski TJ, Coakley TA, Cline SM. Comparison of levalbuterol and racemic albuterol combined with ipratropium bromide in acute pediatric asthma: a randomized controlled trial. Journal of Emergency Medicine 2005;29(1):29‐35. - PubMed
Rayner 1987 {published data only}
Stokes 1983 {published data only}
-
- Stokes GM, Milner AD, Hodges IGC, Henry RL. Nebulised ipratropium bromide in wheezy infants and young children. European Journal of Respiratory Diseases 1983;64:494‐8. - PubMed
Storr 1986 {published data only}
Timsit 2002 {published data only}
-
- Timsit S, Sannier N, Bocquet N, Cojocaru B, Wille C, Boursiquot C, et al. Benefit of ipratropium bromide for the treatment of childhood asthma in the emergency department. Archives de Pediatrie 2002;9(2):117‐24. - PubMed
Vichyanond 1990 {published data only}
-
- Vichyanond P, Sladek WA, Sur S, Hill MR, Szefler SJ, Nelson HS. Efficacy of atropine methylnitrate alone and in combination with albuterol in children with asthma. Chest 1990;98:637‐42. - PubMed
Ward 1981 {published data only}
Ward 1985 {published data only}
-
- Ward MJ, Macfarlane JT, Davies D. A place for ipratropium bromide in the treatment of severe acute asthma. British Journal of Diseases of the Chest 1985;79(4):374‐8. - PubMed
Additional references
Arets 2001
-
- Arets H, Brackel H, Ent C. Forced expiratory manoeuvres in children: do they meet ATS and ERS criteria for spirometry?. European Respiratory Journal 2001;18:655‐60. - PubMed
BTS 2011
-
- British Thoracic Society and Scottish intercollegiate Guidelines Network. British guideline on the management of asthma: a national clinical guideline, 2011. www.brit‐thoracic.org.uk/guidelines (accessed 8 August 2013).
Chalut 2000
-
- Chalut DS, Ducharme FM, Davis GM. The Preschool Respiratory Assessment Measure (PRAM): a responsive index of acute asthma severity. Journal of Pediatrics 2000;137(6):762‐8. - PubMed
Chapman 1996
-
- Chapman K. An international perspective on anticholinergic therapy. American Journal of Medicine 1996;100:2‐4S. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Ducharme 2008
-
- Ducharme FM, Chalut D, Plotnick L, Savadie C, Kudrika D, Zhang Xun, et al. The Pediatric Respiratory Assessment Measure: a valid clinical score for assessing acute asthma severity from toddlers to teenagers. Journal of Pediatrics 2008;152(4):476‐80. - PubMed
GINA 2011
-
- Global INitiative for Asthma. Global strategy for asthma management and prevention, 2011. www.ginasthma.org (accessed 8 August 2013).
Greenland 1985
-
- Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow‐up data. Biometrics 1985;41:55‐68. - PubMed
Gross 1988
-
- Gross NJ. Ipratropium bromide. New England Journal of Medicine 1988;8:486‐94. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Homer 1996
-
- Homer CJ, Szilagyi P, Rodewald L, Bloom SR, Greenspan P, Yazdgerdi S, et al. Does quality of care affect rates of hospitalization for childhood asthma?. Pediatrics 1996;98:18‐23. - PubMed
Khan 1996
-
- Khan KS, Daya S, Jahad AR. The importance of quality of primary studies in producing unbiased systematic reviews. Archives of Internal Medicine 1996;156:661‐6. - PubMed
Lougheed 2012
Payne 1995
-
- Payne SM, Donahue C, Rappo P, McNamara JJ, Bass J, First L, et al. Variations in pediatric pneumonia and bronchitis/asthma admission rates. Is appropriateness a factor?. Archives of Pediatric and Adolescent Medicine 1995;149:162‐9. - PubMed
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration, 2011.
Rodrigo 2005
Rowe 2001
Rubin 2003
-
- Rubin BK, Fink JB. The delivery of inhaled medication to the young child. Pediatric Clinics of North America 2003; Vol. 50, issue 3:717‐31. [0031‐3955: (Print)] - PubMed
Saxena 2006
Sears 1992
-
- Sears MR. Clinical application of beta‐agonists. Practical Allergy and Immunology 1992;7:98‐100.
Silverman 1990
-
- Silverman M. The role of anticholinergic antimuscarinic bronchodilator therapy in children. Lung 1990;168:304‐9. - PubMed
Silverman 2007
-
- Silverman RA, Flaster E, Enright PL, Simonson SG. FEV1 performance among patients with acute asthma: results from a multicenter clinical trial. Chest 2007;131(1):164‐71. - PubMed
Svedmyr 1985
-
- Svedmyr N. A beta2‐adrenergic agonist for use in asthma pharmacology, pharmacokinetics, clinical efficacy and adverse effects. Pharmacotherapy 1985;5:109‐26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

