Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir
- PMID: 23966310
- PMCID: PMC3916776
- DOI: 10.1093/eurheartj/eht331
Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir
Abstract
Aims: Monocytes are critical mediators of healing following acute myocardial infarction (AMI), making them an interesting target to improve myocardial repair. The purpose of this study was a gain of insight into the source and recruitment of monocytes following AMI in humans.
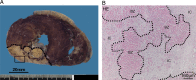
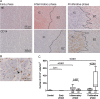
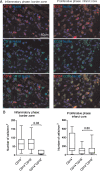
Methods and results: Post-mortem tissue specimens of myocardium, spleen and bone marrow were collected from 28 patients who died at different time points after AMI. Twelve patients who died from other causes served as controls. The presence and localization of monocytes (CD14(+) cells), and their CD14(+)CD16(-) and CD14(+)CD16(+) subsets, were evaluated by immunohistochemical and immunofluorescence analyses. CD14(+) cells localized at distinct regions of the infarcted myocardium in different phases of healing following AMI. In the inflammatory phase after AMI, CD14(+) cells were predominantly located in the infarct border zone, adjacent to cardiomyocytes, and consisted for 85% (78-92%) of CD14(+)CD16(-) cells. In contrast, in the subsequent post-AMI proliferative phase, massive accumulation of CD14(+) cells was observed in the infarct core, containing comparable proportions of both the CD14(+)CD16(-) [60% (31-67%)] and CD14(+)CD16(+) subsets [40% (33-69%)]. Importantly, in AMI patients, of the number of CD14(+) cells was decreased by 39% in the bone marrow and by 58% in the spleen, in comparison with control patients (P = 0.02 and <0.001, respectively).
Conclusions: Overall, this study showed a unique spatiotemporal pattern of monocyte accumulation in the human myocardium following AMI that coincides with a marked depletion of monocytes from the spleen, suggesting that the human spleen contains an important reservoir function for monocytes.
Keywords: Acute myocardial infarction; Bone marrow; Inflammation; Monocytes; Spleen.
Figures


Comment in
-
Immunity strikes: heart failure as a systemic disease.Eur Heart J. 2014 Feb;35(6):341-3. doi: 10.1093/eurheartj/eht405. Epub 2013 Oct 14. Eur Heart J. 2014. PMID: 24126877 No abstract available.
References
-
- Ertl G, Frantz S. Healing after myocardial infarction. Cardiovasc Res. 2005;66:22–32. doi:10.1016/j.cardiores.2005.01.011. - DOI - PubMed
-
- Frantz S, Bauersachs J, Ertl G. Post-infarct remodelling: contribution of wound healing and inflammation. Cardiovasc Res. 2009;81:474–481. doi:10.1093/cvr/cvn292. - DOI - PMC - PubMed
-
- Frangogiannis NG. The mechanistic basis of infarct healing. Antioxid Redox Signal. 2006;8:1907–1939. doi:10.1089/ars.2006.8.1907. - DOI - PubMed
-
- Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88–111. doi:10.1016/j.phrs.2008.06.007. - DOI - PMC - PubMed
-
- Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol. 2008;130:147–158. doi:10.1016/j.ijcard.2008.04.059. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

