Protein kinase C activation decreases peripheral actin network density and increases central nonmuscle myosin II contractility in neuronal growth cones
- PMID: 23966465
- PMCID: PMC3784383
- DOI: 10.1091/mbc.E13-05-0289
Protein kinase C activation decreases peripheral actin network density and increases central nonmuscle myosin II contractility in neuronal growth cones
Abstract
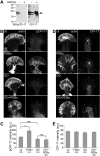
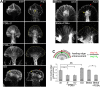
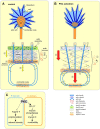
Protein kinase C (PKC) can dramatically alter cell structure and motility via effects on actin filament networks. In neurons, PKC activation has been implicated in repulsive guidance responses and inhibition of axon regeneration; however, the cytoskeletal mechanisms underlying these effects are not well understood. Here we investigate the acute effects of PKC activation on actin network structure and dynamics in large Aplysia neuronal growth cones. We provide evidence of a novel two-tiered mechanism of PKC action: 1) PKC activity enhances myosin II regulatory light chain phosphorylation and C-kinase-potentiated protein phosphatase inhibitor phosphorylation. These effects are correlated with increased contractility in the central cytoplasmic domain. 2) PKC activation results in significant reduction of P-domain actin network density accompanied by Arp2/3 complex delocalization from the leading edge and increased rates of retrograde actin network flow. Our results show that PKC activation strongly affects both actin polymerization and myosin II contractility. This synergistic mode of action is relevant to understanding the pleiotropic reported effects of PKC on neuronal growth and regeneration.
Figures
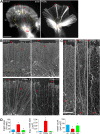
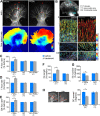

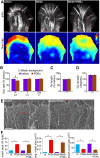
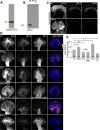

Similar articles
-
Regulation of axon growth by myosin II-dependent mechanocatalysis of cofilin activity.J Cell Biol. 2019 Jul 1;218(7):2329-2349. doi: 10.1083/jcb.201810054. Epub 2019 May 23. J Cell Biol. 2019. PMID: 31123185 Free PMC article.
-
Arp2/3 complex-dependent actin networks constrain myosin II function in driving retrograde actin flow.J Cell Biol. 2012 Jun 25;197(7):939-56. doi: 10.1083/jcb.201111052. Epub 2012 Jun 18. J Cell Biol. 2012. PMID: 22711700 Free PMC article.
-
The role of actin turnover in retrograde actin network flow in neuronal growth cones.PLoS One. 2012;7(2):e30959. doi: 10.1371/journal.pone.0030959. Epub 2012 Feb 16. PLoS One. 2012. PMID: 22359556 Free PMC article.
-
Mammalian nonmuscle myosin II comes in three flavors.Biochem Biophys Res Commun. 2018 Nov 25;506(2):394-402. doi: 10.1016/j.bbrc.2018.03.103. Epub 2018 Mar 17. Biochem Biophys Res Commun. 2018. PMID: 29550471 Free PMC article. Review.
-
The Dynamic Actin Cytoskeleton in Smooth Muscle.Adv Pharmacol. 2018;81:1-38. doi: 10.1016/bs.apha.2017.06.001. Epub 2017 Aug 24. Adv Pharmacol. 2018. PMID: 29310796 Review.
Cited by
-
PKCα diffusion and translocation are independent of an intact cytoskeleton.Sci Rep. 2017 Mar 28;7(1):475. doi: 10.1038/s41598-017-00560-7. Sci Rep. 2017. PMID: 28352102 Free PMC article.
-
Phosphorylation of Nonmuscle myosin II-A regulatory light chain resists Sendai virus fusion with host cells.Sci Rep. 2015 May 20;5:10395. doi: 10.1038/srep10395. Sci Rep. 2015. PMID: 25993465 Free PMC article.
-
Functional interaction of PkcA and PldB regulate aggregation and development in Dictyostelium discoideum.Cell Signal. 2017 Jun;34:47-54. doi: 10.1016/j.cellsig.2017.02.022. Epub 2017 Feb 28. Cell Signal. 2017. PMID: 28257811 Free PMC article.
-
LIM Kinase, a Newly Identified Regulator of Presynaptic Remodeling by Rod Photoreceptors After Injury.Invest Ophthalmol Vis Sci. 2015 Dec;56(13):7847-58. doi: 10.1167/iovs.15-17278. Invest Ophthalmol Vis Sci. 2015. PMID: 26658506 Free PMC article.
-
Depletion of Na+/H+ Exchanger Isoform 1 Increases the Host Cell Resistance to Trypanosoma cruzi Invasion.Pathogens. 2022 Nov 4;11(11):1294. doi: 10.3390/pathogens11111294. Pathogens. 2022. PMID: 36365045 Free PMC article.
References
-
- Allingham JS, Smith R, Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat Struct Mol Biol. 2005;12:378–379. - PubMed
-
- Bement WM, Capco DG. Analysis of inducible contractile rings suggests a role for protein kinase C in embryonic cytokinesis and wound healing. Cell Motil Cytoskeleton. 1991;20:145–157. - PubMed
-
- Bonsall J, Rehder V. Regulation of chick dorsal root ganglion growth cone filopodia by protein kinase C. Brain Res. 1999;839:120–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

