Cooperation between Rho-GEF Gef2 and its binding partner Nod1 in the regulation of fission yeast cytokinesis
- PMID: 23966468
- PMCID: PMC3806657
- DOI: 10.1091/mbc.E13-06-0301
Cooperation between Rho-GEF Gef2 and its binding partner Nod1 in the regulation of fission yeast cytokinesis
Abstract
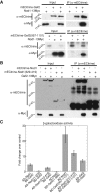
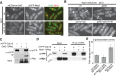
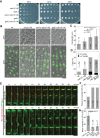
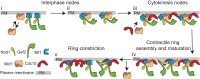
Cytokinesis is the last step of the cell-division cycle, which requires precise spatial and temporal regulation to ensure genetic stability. Rho guanine nucleotide exchange factors (Rho GEFs) and Rho GTPases are among the key regulators of cytokinesis. We previously found that putative Rho-GEF Gef2 coordinates with Polo kinase Plo1 to control the medial cortical localization of anillin-like protein Mid1 in fission yeast. Here we show that an adaptor protein, Nod1, colocalizes with Gef2 in the contractile ring and its precursor cortical nodes. Like gef2, nod1 has strong genetic interactions with various cytokinesis mutants involved in division-site positioning, suggesting a role of Nod1 in early cytokinesis. We find that Nod1 and Gef2 interact through the C-termini, which is important for their localization. The contractile-ring localization of Nod1 and Gef2 also depends on the interaction between Nod1 and the F-BAR protein Cdc15, where the Nod1/Gef2 complex plays a role in contractile-ring maintenance and affects the septation initiation network. Moreover, Gef2 binds to purified GTPases Rho1, Rho4, and Rho5 in vitro. Taken together, our data indicate that Nod1 and Gef2 function cooperatively in a protein complex to regulate fission yeast cytokinesis.
Figures




Similar articles
-
Roles of putative Rho-GEF Gef2 in division-site positioning and contractile-ring function in fission yeast cytokinesis.Mol Biol Cell. 2012 Apr;23(7):1181-95. doi: 10.1091/mbc.E11-09-0800. Epub 2012 Feb 1. Mol Biol Cell. 2012. PMID: 22298427 Free PMC article.
-
Fission yeast Nod1 is a component of cortical nodes involved in cell size control and division site placement.PLoS One. 2013;8(1):e54142. doi: 10.1371/journal.pone.0054142. Epub 2013 Jan 17. PLoS One. 2013. PMID: 23349808 Free PMC article.
-
The Rho-GEF Gef3 interacts with the septin complex and activates the GTPase Rho4 during fission yeast cytokinesis.Mol Biol Cell. 2015 Jan 15;26(2):238-55. doi: 10.1091/mbc.E14-07-1196. Epub 2014 Nov 19. Mol Biol Cell. 2015. PMID: 25411334 Free PMC article.
-
The Multiple Functions of Rho GTPases in Fission Yeasts.Cells. 2021 Jun 7;10(6):1422. doi: 10.3390/cells10061422. Cells. 2021. PMID: 34200466 Free PMC article. Review.
-
Mid1/anillin and the spatial regulation of cytokinesis in fission yeast.Cytoskeleton (Hoboken). 2012 Oct;69(10):764-77. doi: 10.1002/cm.21056. Epub 2012 Aug 20. Cytoskeleton (Hoboken). 2012. PMID: 22888038 Review.
Cited by
-
Processes Controlling the Contractile Ring during Cytokinesis in Fission Yeast, Including the Role of ESCRT Proteins.J Fungi (Basel). 2024 Feb 15;10(2):154. doi: 10.3390/jof10020154. J Fungi (Basel). 2024. PMID: 38392827 Free PMC article. Review.
-
Roles of the fission yeast UNC-13/Munc13 protein Ync13 in late stages of cytokinesis.Mol Biol Cell. 2018 Sep 15;29(19):2259-2279. doi: 10.1091/mbc.E18-04-0225. Epub 2018 Jul 25. Mol Biol Cell. 2018. PMID: 30044717 Free PMC article.
-
Roles of Mso1 and the SM protein Sec1 in efficient vesicle fusion during fission yeast cytokinesis.Mol Biol Cell. 2020 Jul 15;31(15):1570-1583. doi: 10.1091/mbc.E20-01-0067. Epub 2020 May 20. Mol Biol Cell. 2020. PMID: 32432970 Free PMC article.
-
Regulation of Rho-GEF Rgf3 by the arrestin Art1 in fission yeast cytokinesis.Mol Biol Cell. 2015 Feb 1;26(3):453-66. doi: 10.1091/mbc.E14-07-1252. Epub 2014 Dec 3. Mol Biol Cell. 2015. PMID: 25473118 Free PMC article.
-
Roles of the novel coiled-coil protein Rng10 in septum formation during fission yeast cytokinesis.Mol Biol Cell. 2016 Aug 15;27(16):2528-41. doi: 10.1091/mbc.E16-03-0156. Epub 2016 Jul 6. Mol Biol Cell. 2016. PMID: 27385337 Free PMC article.
References
-
- Almonacid M, Moseley JB, Janvore J, Mayeux A, Fraisier V, Nurse P, Paoletti A. Spatial control of cytokinesis by Cdr2 kinase and Mid1/anillin nuclear export. Curr Biol. 2009;19:961–966. - PubMed
-
- Arellano M, Duran A, Pérez P. Localisation of the Schizosaccharomyces pombe rho1p GTPase and its involvement in the organisation of the actin cytoskeleton. J Cell Sci. 1997;110:2547–2555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

