Tissue-specific and SRSF1-dependent splicing of fibronectin, a matrix protein that controls host cell invasion
- PMID: 23966470
- PMCID: PMC3806663
- DOI: 10.1091/mbc.E13-03-0142
Tissue-specific and SRSF1-dependent splicing of fibronectin, a matrix protein that controls host cell invasion
Abstract
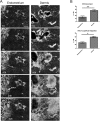
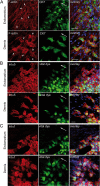
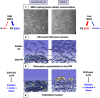
Cell invasion targets specific tissues in physiological placental implantation and pathological metastasis, which raises questions about how this process is controlled. We compare dermis and endometrium capacities to support trophoblast invasion, using matching sets of human primary fibroblasts in a coculture assay with human placental explants. Substituting endometrium, the natural trophoblast target, with dermis dramatically reduces trophoblast interstitial invasion. Our data reveal that endometrium expresses a higher rate of the fibronectin (FN) extra type III domain A+ (EDA+) splicing isoform, which displays stronger matrix incorporation capacity. We demonstrate that the high FN content of the endometrium matrix, and not specifically the EDA domain, supports trophoblast invasion by showing that forced incorporation of plasma FN (EDA-) promotes efficient trophoblast invasion. We further show that the serine/arginine-rich protein serine/arginine-rich splicing factor 1 (SRSF1) is more highly expressed in endometrium and, using RNA interference, that it is involved in the higher EDA exon inclusion rate in endometrium. Our data therefore show a mechanism by which tissues can be distinguished, for their capacity to support invasion, by their different rates of EDA inclusion, linked to their SRSF1 protein levels. In the broader context of cancer pathology, the results suggest that SRSF1 might play a central role not only in the tumor cells, but also in the surrounding stroma.
Figures







Similar articles
-
Emerging functions of SRSF1, splicing factor and oncoprotein, in RNA metabolism and cancer.Mol Cancer Res. 2014 Sep;12(9):1195-204. doi: 10.1158/1541-7786.MCR-14-0131. Epub 2014 May 7. Mol Cancer Res. 2014. PMID: 24807918 Free PMC article. Review.
-
Formation of an invasion-permissive matrix requires TGFβ/SNAIL1-regulated alternative splicing of fibronectin.Breast Cancer Res. 2023 Nov 14;25(1):143. doi: 10.1186/s13058-023-01736-y. Breast Cancer Res. 2023. PMID: 37964360 Free PMC article.
-
Transforming growth factor-beta1 regulates fibronectin isoform expression and splicing factor SRp40 expression during ATDC5 chondrogenic maturation.Exp Cell Res. 2007 May 1;313(8):1518-32. doi: 10.1016/j.yexcr.2007.01.028. Epub 2007 Feb 28. Exp Cell Res. 2007. PMID: 17391668 Free PMC article.
-
TGFβ1-mediated PI3K/Akt and p38 MAP kinase dependent alternative splicing of fibronectin extra domain A in human podocyte culture.Cell Mol Biol (Noisy-le-grand). 2018 Apr 30;64(5):127-135. Cell Mol Biol (Noisy-le-grand). 2018. PMID: 29729706
-
Regulation of placentation by the transforming growth factor beta superfamily†.Biol Reprod. 2020 Feb 12;102(1):18-26. doi: 10.1093/biolre/ioz186. Biol Reprod. 2020. PMID: 31566220 Review.
Cited by
-
Extravillous trophoblasts reverse the decidualization induced increase in matrix production by secreting TGFβ antagonists Emilin-1 and Gremlin-1.Cells Dev. 2025 Mar;181:203994. doi: 10.1016/j.cdev.2025.203994. Epub 2025 Jan 3. Cells Dev. 2025. PMID: 39756583
-
Regulation of Placental Extravillous Trophoblasts by the Maternal Uterine Environment.Front Immunol. 2018 Nov 13;9:2597. doi: 10.3389/fimmu.2018.02597. eCollection 2018. Front Immunol. 2018. PMID: 30483261 Free PMC article. Review.
-
Extracellular Matrix-Derived Damage-Associated Molecular Patterns (DAMP): Implications in Systemic Sclerosis and Fibrosis.J Invest Dermatol. 2023 Oct;143(10):1877-1885. doi: 10.1016/j.jid.2023.04.030. Epub 2023 Jul 14. J Invest Dermatol. 2023. PMID: 37452808 Free PMC article. Review.
-
Emerging functions of SRSF1, splicing factor and oncoprotein, in RNA metabolism and cancer.Mol Cancer Res. 2014 Sep;12(9):1195-204. doi: 10.1158/1541-7786.MCR-14-0131. Epub 2014 May 7. Mol Cancer Res. 2014. PMID: 24807918 Free PMC article. Review.
-
Best evidence linking the extracellular factor TGF-β to cancer-associated alternative splicing programs.BBA Adv. 2024 Dec 18;7:100132. doi: 10.1016/j.bbadva.2024.100132. eCollection 2025. BBA Adv. 2024. PMID: 40585016 Free PMC article. Review.
References
-
- Abe Y, Bui-Thanh NA, Ballantyne CM, Burns AR. Extra domain A and type III connecting segment of fibronectin in assembly and cleavage. Biochem Biophys Res Commun. 2005;338:1640–1647. - PubMed
-
- Aplin JD, Haigh T, Jones CJ, Church HJ, Vicovac L. Development of cytotrophoblast columns from explanted first-trimester human placental villi: role of fibronectin and integrin alpha5beta1. Biol Reprod. 1999;60:828–838. - PubMed
-
- Barash Y, Calarco JA, Gao W, Pan Q, Wang X, Shai O, Blencowe BJ, Frey BJ. Deciphering the splicing code. Nature. 2010;465:53–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

