Short- and long-term plasticity in CA1 neurons from mice lacking h-channel auxiliary subunit TRIP8b
- PMID: 23966674
- PMCID: PMC3841871
- DOI: 10.1152/jn.00218.2013
Short- and long-term plasticity in CA1 neurons from mice lacking h-channel auxiliary subunit TRIP8b
Abstract
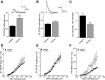
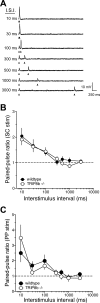
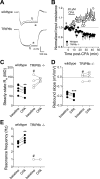
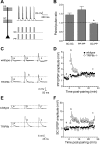
Hyperpolarization-activated cyclic nucleotide-gated nonselective cation channels (HCN or h-channels) are important regulators of neuronal physiology contributing to passive membrane properties, such as resting membrane potential and input resistance (R(N)), and to intrinsic oscillatory activity and synaptic integration. The correct membrane targeting of h-channels is regulated in part by the auxiliary h-channel protein TRIP8b. The genetic deletion of TRIP8b results in a loss of functional h-channels, which affects the postsynaptic integrative properties of neurons. We investigated the impact of TRIP8b deletion on long-term potentiation (LTP) at the two major excitatory inputs to CA1 pyramidal neurons: Schaffer collateral (SC) and perforant path (PP). We found that SC LTP was not significantly different between neurons from wild-type and TRIP8b-knockout mice. There was, however, significantly more short-term potentiation in knockout neurons. We also found that the persistent increase in h-current (I(h)) that normally occurs after LTP induction was absent in knockout neurons. The lack of I(h) plasticity was not restricted to activity-dependent induction, because the depletion of intracellular calcium stores also failed to produce the expected increase in I(h). Interestingly, pairing of SC and PP inputs resulted in a form of LTP in knockout neurons that did not occur in wild-type neurons. These results suggest that the physiological impact of TRIP8b deletion is not restricted to the integrative properties of neurons but also includes both synaptic and intrinsic plasticity.
Keywords: Ih; Schaffer collateral; hippocampus; intrinsic plasticity; perforant path.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

