Reliable sex and strain discrimination in the mouse vomeronasal organ and accessory olfactory bulb
- PMID: 23966710
- PMCID: PMC3755725
- DOI: 10.1523/JNEUROSCI.0037-13.2013
Reliable sex and strain discrimination in the mouse vomeronasal organ and accessory olfactory bulb
Abstract
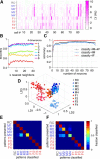
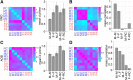
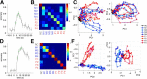
Animals modulate their courtship and territorial behaviors in response to olfactory cues produced by other animals. In rodents, detecting these cues is the primary role of the accessory olfactory system (AOS). We sought to systematically investigate the natural stimulus coding logic and robustness in neurons of the first two stages of accessory olfactory processing, the vomeronasal organ (VNO) and accessory olfactory bulb (AOB). We show that firing rate responses of just a few well-chosen mouse VNO or AOB neurons can be used to reliably encode both sex and strain of other mice from cues contained in urine. Additionally, we show that this population code can generalize to new concentrations of stimuli and appears to represent stimulus identity in terms of diverging paths in coding space. Together, the results indicate that firing rate code on the temporal order of seconds is sufficient for accurate classification of pheromonal patterns at different concentrations and may be used by AOS neural circuitry to discriminate among naturally occurring urine stimuli.
Figures






Similar articles
-
Temporal Response Properties of Accessory Olfactory Bulb Neurons: Limitations and Opportunities for Decoding.J Neurosci. 2018 May 23;38(21):4957-4976. doi: 10.1523/JNEUROSCI.2091-17.2018. Epub 2018 Apr 30. J Neurosci. 2018. PMID: 29712784 Free PMC article.
-
Encoding pheromonal signals in the accessory olfactory bulb of behaving mice.Science. 2003 Feb 21;299(5610):1196-201. doi: 10.1126/science.1082133. Science. 2003. PMID: 12595684
-
In vivo vomeronasal stimulation reveals sensory encoding of conspecific and allospecific cues by the mouse accessory olfactory bulb.Proc Natl Acad Sci U S A. 2010 Mar 16;107(11):5172-7. doi: 10.1073/pnas.0915147107. Epub 2010 Mar 1. Proc Natl Acad Sci U S A. 2010. PMID: 20194746 Free PMC article.
-
Histological properties of the glomerular layer in the mouse accessory olfactory bulb.Exp Anim. 2012;61(1):13-24. doi: 10.1538/expanim.61.13. Exp Anim. 2012. PMID: 22293668 Review.
-
Encoding pheromonal signals in the mammalian vomeronasal system.Curr Opin Neurobiol. 2004 Aug;14(4):428-34. doi: 10.1016/j.conb.2004.07.001. Curr Opin Neurobiol. 2004. PMID: 15321063 Review.
Cited by
-
Signal Detection and Coding in the Accessory Olfactory System.Chem Senses. 2018 Nov 1;43(9):667-695. doi: 10.1093/chemse/bjy061. Chem Senses. 2018. PMID: 30256909 Free PMC article. Review.
-
Cupid's quiver: Integrating sensory cues in rodent mating systems.Front Neural Circuits. 2022 Jul 25;16:944895. doi: 10.3389/fncir.2022.944895. eCollection 2022. Front Neural Circuits. 2022. PMID: 35958042 Free PMC article. Review.
-
A Molecular Code for Identity in the Vomeronasal System.Cell. 2015 Oct 8;163(2):313-23. doi: 10.1016/j.cell.2015.09.012. Epub 2015 Oct 1. Cell. 2015. PMID: 26435105 Free PMC article.
-
Limbic Neurons Shape Sex Recognition and Social Behavior in Sexually Naive Males.Cell. 2019 Feb 21;176(5):1190-1205.e20. doi: 10.1016/j.cell.2018.12.041. Epub 2019 Jan 31. Cell. 2019. PMID: 30712868 Free PMC article.
-
Ex vivo preparations of the intact vomeronasal organ and accessory olfactory bulb.J Vis Exp. 2014 Aug 4;(90):e51813. doi: 10.3791/51813. J Vis Exp. 2014. PMID: 25145699 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
