Optogenetic control of fly optomotor responses
- PMID: 23966712
- PMCID: PMC6618655
- DOI: 10.1523/JNEUROSCI.0340-13.2013
Optogenetic control of fly optomotor responses
Abstract
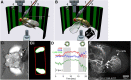
When confronted with a large-field stimulus rotating around the vertical body axis, flies display a following behavior called "optomotor response." As neural control elements, the large tangential horizontal system (HS) cells of the lobula plate have been prime candidates for long. Here, we applied optogenetic stimulation of HS cells to evaluate their behavioral role in Drosophila. To minimize interference of the optical activation of channelrhodopsin-2 with the visual perception of the flies, we used a bistable variant called ChR2-C128S. By applying pulses of blue and yellow light, we first demonstrate electrophysiologically that lobula plate tangential cells can be activated and deactivated repeatedly with no evident change in depolarization strength over trials. We next show that selective optogenetic activation of HS cells elicits robust yaw head movements and yaw turning responses in fixed and tethered flying flies, respectively.
Figures



References
-
- Bishop LG, Keehn DG. Neural correlates of the optomotor responses in the fly. Kybernetik. 1967;3:288–295. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
