Simulating cortical development as a self constructing process: a novel multi-scale approach combining molecular and physical aspects
- PMID: 23966845
- PMCID: PMC3744399
- DOI: 10.1371/journal.pcbi.1003173
Simulating cortical development as a self constructing process: a novel multi-scale approach combining molecular and physical aspects
Erratum in
-
Erratum: Notice of Republication: Simulating Cortical Development as a Self Constructing Process: A Novel Multi-Scale Approach Combining Molecular and Physical Aspects.PLoS Comput Biol. 2019 Jan 23;15(1):e1006747. doi: 10.1371/journal.pcbi.1006747. eCollection 2019 Jan. PLoS Comput Biol. 2019. PMID: 30673692 Free PMC article.
Abstract
Current models of embryological development focus on intracellular processes such as gene expression and protein networks, rather than on the complex relationship between subcellular processes and the collective cellular organization these processes support. We have explored this collective behavior in the context of neocortical development, by modeling the expansion of a small number of progenitor cells into a laminated cortex with layer and cell type specific projections. The developmental process is steered by a formal language analogous to genomic instructions, and takes place in a physically realistic three-dimensional environment. A common genome inserted into individual cells control their individual behaviors, and thereby gives rise to collective developmental sequences in a biologically plausible manner. The simulation begins with a single progenitor cell containing the artificial genome. This progenitor then gives rise through a lineage of offspring to distinct populations of neuronal precursors that migrate to form the cortical laminae. The precursors differentiate by extending dendrites and axons, which reproduce the experimentally determined branching patterns of a number of different neuronal cell types observed in the cat visual cortex. This result is the first comprehensive demonstration of the principles of self-construction whereby the cortical architecture develops. In addition, our model makes several testable predictions concerning cell migration and branching mechanisms.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

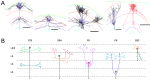
 . (Reconstructed cells are a courtesy of Kevan Martin and colleagues, Institute of Neuroinformatics, Zurich). (B) Schematic representation of the axonal branching pattern for encoding in the instruction language G-code. Each color corresponds to a segment of the axonal arbor encoded by a specific G-code machine.
. (Reconstructed cells are a courtesy of Kevan Martin and colleagues, Institute of Neuroinformatics, Zurich). (B) Schematic representation of the axonal branching pattern for encoding in the instruction language G-code. Each color corresponds to a segment of the axonal arbor encoded by a specific G-code machine.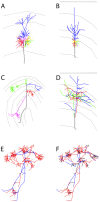
 are in red, the segments with fewer boutons are in blue. (F) The differentiation between backbone and patches based on the branching angles is less obvious. Segments displayed in black make an angle
are in red, the segments with fewer boutons are in blue. (F) The differentiation between backbone and patches based on the branching angles is less obvious. Segments displayed in black make an angle  degrees to the parent branch (corresponding in principle to side branches); for segments in red the angle with the parent branch is 20–80 degrees (corresponding in principle to bifurcation); the segments in blue are continuations of the mother branch (angle
degrees to the parent branch (corresponding in principle to side branches); for segments in red the angle with the parent branch is 20–80 degrees (corresponding in principle to bifurcation); the segments in blue are continuations of the mother branch (angle  degrees; continuation of a major shaft). Reconstructed cells are courtesy of Kevan Martin and colleagues, Institute of Neuroinformatics, Zurich.
degrees; continuation of a major shaft). Reconstructed cells are courtesy of Kevan Martin and colleagues, Institute of Neuroinformatics, Zurich.

References
-
- Kitano H (2002) Systems biology: a brief overview. Science 295: 1662–1664. - PubMed
-
- Alon U (2006) An Introduction to Systems Biology: Design Principles of Biological Circuits. Chapman & Hall/CRC.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

