Coronaviruses lacking exoribonuclease activity are susceptible to lethal mutagenesis: evidence for proofreading and potential therapeutics
- PMID: 23966862
- PMCID: PMC3744431
- DOI: 10.1371/journal.ppat.1003565
Coronaviruses lacking exoribonuclease activity are susceptible to lethal mutagenesis: evidence for proofreading and potential therapeutics
Erratum in
- PLoS Pathog. 2014 Jul;10(7):e1004342. Surdel, Matthew C [added]
Abstract
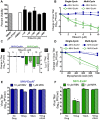
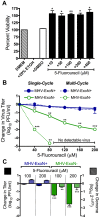
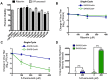
No therapeutics or vaccines currently exist for human coronaviruses (HCoVs). The Severe Acute Respiratory Syndrome-associated coronavirus (SARS-CoV) epidemic in 2002-2003, and the recent emergence of Middle East Respiratory Syndrome coronavirus (MERS-CoV) in April 2012, emphasize the high probability of future zoonotic HCoV emergence causing severe and lethal human disease. Additionally, the resistance of SARS-CoV to ribavirin (RBV) demonstrates the need to define new targets for inhibition of CoV replication. CoVs express a 3'-to-5' exoribonuclease in nonstructural protein 14 (nsp14-ExoN) that is required for high-fidelity replication and is conserved across the CoV family. All genetic and biochemical data support the hypothesis that nsp14-ExoN has an RNA proofreading function. Thus, we hypothesized that ExoN is responsible for CoV resistance to RNA mutagens. We demonstrate that while wild-type (ExoN+) CoVs were resistant to RBV and 5-fluorouracil (5-FU), CoVs lacking ExoN activity (ExoN-) were up to 300-fold more sensitive. While the primary antiviral activity of RBV against CoVs was not mutagenesis, ExoN- CoVs treated with 5-FU demonstrated both enhanced sensitivity during multi-cycle replication, as well as decreased specific infectivity, consistent with 5-FU functioning as a mutagen. Comparison of full-genome next-generation sequencing of 5-FU treated SARS-CoV populations revealed a 16-fold increase in the number of mutations within the ExoN- population as compared to ExoN+. Ninety percent of these mutations represented A:G and U:C transitions, consistent with 5-FU incorporation during RNA synthesis. Together our results constitute direct evidence that CoV ExoN activity provides a critical proofreading function during virus replication. Furthermore, these studies identify ExoN as the first viral protein distinct from the RdRp that determines the sensitivity of RNA viruses to mutagens. Finally, our results show the importance of ExoN as a target for inhibition, and suggest that small-molecule inhibitors of ExoN activity could be potential pan-CoV therapeutics in combination with RBV or RNA mutagens.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Comment in
-
Viral evolution: fooling the coronavirus proofreading machinery.Nat Rev Microbiol. 2013 Oct;11(10):662-3. doi: 10.1038/nrmicro3125. Epub 2013 Sep 10. Nat Rev Microbiol. 2013. PMID: 24018385 Free PMC article.
References
-
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA (2012) Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 367: 1814–1820. - PubMed
-
- Drosten C, Gunther S, Preiser W, van der Werf S, Brodt HR, et al. (2003) Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 348: 1967–1976. - PubMed
-
- Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, et al. (2003) A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 348: 1953–1966. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

