Mechanism based approaches for rescuing and enhancing cognition
- PMID: 23966908
- PMCID: PMC3744010
- DOI: 10.3389/fnins.2013.00143
Mechanism based approaches for rescuing and enhancing cognition
Abstract
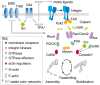
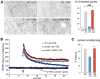
Progress toward pharmacological means for enhancing memory and cognition has been retarded by the widely discussed failure of behavioral studies in animals to predict human outcomes. As a result, a number of groups have targeted cognition-related neurobiological mechanisms in animal models, with the assumption that these basic processes are highly conserved across mammals. Here we survey one such approach that begins with a form of synaptic plasticity intimately related to memory encoding in animals and likely operative in humans. An initial section will describe a detailed hypothesis concerning the signaling and structural events (a "substrate map") that convert learning associated patterns of afferent activity into extremely stable increases in fast, excitatory transmission. We next describe results suggesting that all instances of intellectual impairment so far tested in rodent models involve a common endpoint failure in the substrate map. This will be followed by a clinically plausible proposal for obviating the ultimate defect in these models. We then take up the question of whether it is reasonable to expect, from either general principles or a very limited set of experimental results, that enhancing memory will expand the cognitive capabilities of high functioning brains. The final section makes several suggestions about how to improve translation of behavioral results from animals to humans. Collectively, the material covered here points to the following: (1) enhancement, in the sense of rescue, is not an unrealistic possibility for a broad array of neuropsychiatric disorders; (2) serendipity aside, developing means for improving memory in normals will likely require integration of information about mechanisms with new behavioral testing strategies; (3) a shift in emphasis from synapses to networks is a next, logical step in the evolution of the cognition enhancement field.
Keywords: LTP; ampakine; animal models; cognitive enhancement; cytoskeleton; learning; long-term potentiation; spaced trials.
Figures


References
-
- Arai A., Kessler M., Rogers G., Lynch G. (1996). Effects of a memory enhancing drug on AMPA receptor currents and synaptic transmission in hippocampus. J. Pharmacol. Exp. Ther. 278, 627–638 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

