Improved methods for chronic light-based motor mapping in mice: automated movement tracking with accelerometers, and chronic EEG recording in a bilateral thin-skull preparation
- PMID: 23966910
- PMCID: PMC3722499
- DOI: 10.3389/fncir.2013.00123
Improved methods for chronic light-based motor mapping in mice: automated movement tracking with accelerometers, and chronic EEG recording in a bilateral thin-skull preparation
Abstract
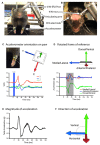
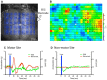
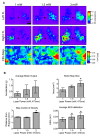
Optogenetic stimulation of the mouse cortex can be used to generate motor maps that are similar to maps derived from electrode-based stimulation. Here we present a refined set of procedures for repeated light-based motor mapping in ChR2-expressing mice implanted with a bilateral thinned-skull chronic window and a chronically implanted electroencephalogram (EEG) electrode. Light stimulation is delivered sequentially to over 400 points across the cortex, and evoked movements are quantified on-line with a three-axis accelerometer attached to each forelimb. Bilateral maps of forelimb movement amplitude and movement direction were generated at weekly intervals after recovery from cranial window implantation. We found that light pulses of ~2 mW produced well-defined maps that were centered approximately 0.7 mm anterior and 1.6 mm lateral from bregma. Map borders were defined by sites where light stimulation evoked EEG deflections, but not movements. Motor maps were similar in size and location between mice, and maps were stable over weeks in terms of the number of responsive sites, and the direction of evoked movements. We suggest that our method may be used to chronically assess evoked motor output in mice, and may be combined with other imaging tools to assess cortical reorganization or sensory-motor integration.
Keywords: accelerometer; chronic EEG; chronic window; cortex; motor mapping; mouse; optogenetics.
Figures


References
-
- Asanuma H., Sakata H. (1967) Functional organization of a cortical efferent system examined with focal depth stimulation in cats. J. Neurophysiol. 30 35–54
-
- Bonazzi L., Viaro R., Lodi E., Canto R., Bonifazzi C., Franchi G. (2013) Complex movement topography and extrinsic space representation in the rat forelimb motor cortex as defined by long-duration intracortical microstimulation. J. Neurosci. 33 2097–2107 10.1523/JNEUROSCI.3454-12.2013 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

