Intracellular reprogramming of expression, glycosylation, and function of a plant-derived antiviral therapeutic monoclonal antibody
- PMID: 23967055
- PMCID: PMC3744537
- DOI: 10.1371/journal.pone.0068772
Intracellular reprogramming of expression, glycosylation, and function of a plant-derived antiviral therapeutic monoclonal antibody
Abstract
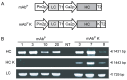
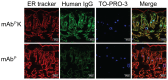
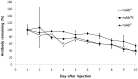
Plant genetic engineering, which has led to the production of plant-derived monoclonal antibodies (mAb(P)s), provides a safe and economically effective alternative to conventional antibody expression methods. In this study, the expression levels and biological properties of the anti-rabies virus mAb(P) SO57 with or without an endoplasmic reticulum (ER)-retention peptide signal (Lys-Asp-Glu-Leu; KDEL) in transgenic tobacco plants (Nicotiana tabacum) were analyzed. The expression levels of mAb(P) SO57 with KDEL (mAb(P)K) were significantly higher than those of mAb(P) SO57 without KDEL (mAb(P)) regardless of the transcription level. The Fc domains of both purified mAb(P) and mAb(P)K and hybridoma-derived mAb (mAb(H)) had similar levels of binding activity to the FcγRI receptor (CD64). The mAb(P)K had glycan profiles of both oligomannose (OM) type (91.7%) and Golgi type (8.3%), whereas the mAb(P) had mainly Golgi type glycans (96.8%) similar to those seen with mAb(H). Confocal analysis showed that the mAb(P)K was co-localized to ER-tracker signal and cellular areas surrounding the nucleus indicating accumulation of the mAb(P) with KDEL in the ER. Both mAb(P) and mAb(P)K disappeared with similar trends to mAb(H) in BALB/c mice. In addition, mAb(P)K was as effective as mAb(H) at neutralizing the activity of the rabies virus CVS-11. These results suggest that the ER localization of the recombinant mAb(P) by KDEL reprograms OM glycosylation and enhances the production of the functional antivirus therapeutic antibody in the plant.
Conflict of interest statement
Figures

Similar articles
-
Differential N-glycosylation of a monoclonal antibody expressed in tobacco leaves with and without endoplasmic reticulum retention signal apparently induces similar in vivo stability in mice.Plant Biotechnol J. 2011 Dec;9(9):1120-30. doi: 10.1111/j.1467-7652.2011.00638.x. Epub 2011 Aug 7. Plant Biotechnol J. 2011. PMID: 21819534
-
Fc engineered anti-virus therapeutic human IgG1 expressed in plants with altered binding to the neonatal Fc receptor.Biotechnol J. 2024 Mar;19(3):e2300552. doi: 10.1002/biot.202300552. Biotechnol J. 2024. PMID: 38528347
-
Controlled glycosylation of therapeutic antibodies in plants.Arch Biochem Biophys. 2004 Jun 15;426(2):266-78. doi: 10.1016/j.abb.2004.02.034. Arch Biochem Biophys. 2004. PMID: 15158677
-
Manufacturing antibodies in the plant cell.Biotechnol J. 2009 Dec;4(12):1712-24. doi: 10.1002/biot.200900223. Biotechnol J. 2009. PMID: 20014227 Review.
-
Secretory IgA antibodies from plants.Curr Pharm Des. 2005;11(19):2429-37. doi: 10.2174/1381612054367508. Curr Pharm Des. 2005. PMID: 16026297 Review.
Cited by
-
Current Developments and Future Prospects for Plant-Made Biopharmaceuticals Against Rabies.Mol Biotechnol. 2015 Oct;57(10):869-79. doi: 10.1007/s12033-015-9880-3. Mol Biotechnol. 2015. PMID: 26163274 Review.
-
N-glycosylation modification of plant-derived virus-like particles: an application in vaccines.Biomed Res Int. 2014;2014:249519. doi: 10.1155/2014/249519. Epub 2014 May 25. Biomed Res Int. 2014. PMID: 24971324 Free PMC article. Review.
-
Biotechnologically Engineered Plants.Biology (Basel). 2023 Apr 15;12(4):601. doi: 10.3390/biology12040601. Biology (Basel). 2023. PMID: 37106801 Free PMC article. Review.
-
Live-cell imaging of the chloroplast outer envelope membrane using fluorescent dyes.Plant Direct. 2022 Nov 15;6(11):e462. doi: 10.1002/pld3.462. eCollection 2022 Nov. Plant Direct. 2022. PMID: 36398034 Free PMC article.
-
Effect of an Endoplasmic Reticulum Retention Signal Tagged to Human Anti-Rabies mAb SO57 on Its Expression in Arabidopsis and Plant Growth.Mol Cells. 2021 Oct 31;44(10):770-779. doi: 10.14348/molcells.2021.2002. Mol Cells. 2021. PMID: 34711693 Free PMC article.
References
-
- Cliquet F, Gurbuxani JP, Pradhan HK, Pattnaik B, Patil SS, et al. (2007) The safety and efficacy of the oral rabies vaccine SAG2 in Indian stray dogs. Vaccine 25: 3409–3418. - PubMed
-
- Hu R, Zhang S, Fooks AR, Yuan H, Liu Y, et al. (2006) Prevention of rabies virus infection in dogs by a recombinant canine adenovirus type-2 encoding the rabies virus glycoprotein. Microbes and Infection 8: 1090–1097. - PubMed
-
- Wilde H, Thipkon P, Sitprija V, Chaiyabutr N (1996) Heterologous Antisera and Antivenins Are Essential Biologicals: Perspectives on a Worldwide Crisis. Ann Intern Med 125: 233–236. - PubMed
-
- Richter LJ, Thanavala Y, Arntzen CJ, Mason HS (2000) Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nat Biotechnol 18: 1167–1171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

