L-arginine and vitamin D adjunctive therapies in pulmonary tuberculosis: a randomised, double-blind, placebo-controlled trial
- PMID: 23967066
- PMCID: PMC3743888
- DOI: 10.1371/journal.pone.0070032
L-arginine and vitamin D adjunctive therapies in pulmonary tuberculosis: a randomised, double-blind, placebo-controlled trial
Abstract
Background: Vitamin D (vitD) and L-arginine have important antimycobacterial effects in humans. Adjunctive therapy with these agents has the potential to improve outcomes in active tuberculosis (TB).
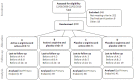
Methods: In a 4-arm randomised, double-blind, placebo-controlled factorial trial in adults with smear-positive pulmonary tuberculosis (PTB) in Timika, Indonesia, we tested the effect of oral adjunctive vitD 50,000 IU 4-weekly or matching placebo, and L-arginine 6.0 g daily or matching placebo, for 8 weeks, on proportions of participants with negative 4-week sputum culture, and on an 8-week clinical score (weight, FEV1, cough, sputum, haemoptysis). All participants with available endpoints were included in analyses according to the study arm to which they were originally assigned. Adults with new smear-positive PTB were eligible. The trial was registered at ClinicalTrials.gov NCT00677339.
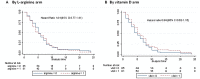
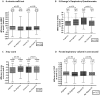
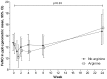
Results: 200 participants were enrolled, less than the intended sample size: 50 received L-arginine + active vitD, 49 received L-arginine + placebo vit D, 51 received placebo L-arginine + active vitD and 50 received placebo L-arginine + placebo vitD. According to the factorial model, 99 people received arginine, 101 placebo arginine, 101 vitamin D, 99 placebo vitamin D. Results for the primary endpoints were available in 155 (4-week culture) and 167 (clinical score) participants. Sputum culture conversion was achieved by week 4 in 48/76 (63%) participants in the active L-arginine versus 48/79 (61%) in placebo L-arginine arms (risk difference -3%, 95% CI -19 to 13%), and in 44/75 (59%) in the active vitD versus 52/80 (65%) in the placebo vitD arms (risk difference 7%, 95% CI -9 to 22%). The mean clinical outcome score also did not differ between study arms. There were no effects of the interventions on adverse event rates including hypercalcaemia, or other secondary outcomes.
Conclusion: Neither vitD nor L-arginine supplementation, at the doses administered and with the power attained, affected TB outcomes.
Registry: ClinicalTrials.gov. Registry number: NCT00677339.
Conflict of interest statement
Figures

References
-
- Onyebujoh P, Rodriguez W, Mwaba P (2006) Priorities in tuberculosis research. Lancet 367: 940–942. - PubMed
-
- Sinclair D, Abba K, Grobler L, Sudarsanam TD (2011) Nutritional supplements for people being treated for active tuberculosis. Cochrane Database Syst Rev CD006086. - PubMed
-
- Ralph AP, Kelly PM, Anstey NM (2008) L-arginine and vitamin D: novel adjunctive immunotherapies in tuberculosis. Trends Microbiol 16: 336–344. - PubMed
-
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, et al. (2006) Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311: 1770–1773. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

