Identification of multiple subsets of ventral interneurons and differential distribution along the rostrocaudal axis of the developing spinal cord
- PMID: 23967072
- PMCID: PMC3744532
- DOI: 10.1371/journal.pone.0070325
Identification of multiple subsets of ventral interneurons and differential distribution along the rostrocaudal axis of the developing spinal cord
Abstract
The spinal cord contains neuronal circuits termed Central Pattern Generators (CPGs) that coordinate rhythmic motor activities. CPG circuits consist of motor neurons and multiple interneuron cell types, many of which are derived from four distinct cardinal classes of ventral interneurons, called V0, V1, V2 and V3. While significant progress has been made on elucidating the molecular and genetic mechanisms that control ventral interneuron differentiation, little is known about their distribution along the antero-posterior axis of the spinal cord and their diversification. Here, we report that V0, V1 and V2 interneurons exhibit distinct organizational patterns at brachial, thoracic and lumbar levels of the developing spinal cord. In addition, we demonstrate that each cardinal class of ventral interneurons can be subdivided into several subsets according to the combinatorial expression of different sets of transcription factors, and that these subsets are differentially distributed along the rostrocaudal axis of the spinal cord. This comprehensive molecular profiling of ventral interneurons provides an important resource for investigating neuronal diversification in the developing spinal cord and for understanding the contribution of specific interneuron subsets on CPG circuits and motor control.
Conflict of interest statement
Figures

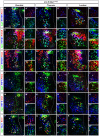

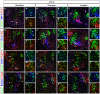

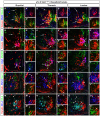

Similar articles
-
Postnatal phenotype and localization of spinal cord V1 derived interneurons.J Comp Neurol. 2005 Dec 12;493(2):177-92. doi: 10.1002/cne.20711. J Comp Neurol. 2005. PMID: 16255029 Free PMC article.
-
Evx1 is a postmitotic determinant of v0 interneuron identity in the spinal cord.Neuron. 2001 Feb;29(2):385-99. doi: 10.1016/s0896-6273(01)00213-6. Neuron. 2001. PMID: 11239430
-
Genetic dissection of Gata2 selective functions during specification of V2 interneurons in the developing spinal cord.Dev Neurobiol. 2015 Jul;75(7):721-37. doi: 10.1002/dneu.22244. Epub 2014 Nov 15. Dev Neurobiol. 2015. PMID: 25369423
-
Spinal Inhibitory Interneurons: Gatekeepers of Sensorimotor Pathways.Int J Mol Sci. 2021 Mar 6;22(5):2667. doi: 10.3390/ijms22052667. Int J Mol Sci. 2021. PMID: 33800863 Free PMC article. Review.
-
New perspectives on the mechanisms establishing the dorsal-ventral axis of the spinal cord.Curr Top Dev Biol. 2019;132:417-450. doi: 10.1016/bs.ctdb.2018.12.010. Epub 2018 Dec 26. Curr Top Dev Biol. 2019. PMID: 30797516 Free PMC article. Review.
Cited by
-
Potential contribution of spinal interneurons to the etiopathogenesis of amyotrophic lateral sclerosis.Front Neurosci. 2024 Jul 18;18:1434404. doi: 10.3389/fnins.2024.1434404. eCollection 2024. Front Neurosci. 2024. PMID: 39091344 Free PMC article. Review.
-
Vsx1 Transiently Defines an Early Intermediate V2 Interneuron Precursor Compartment in the Mouse Developing Spinal Cord.Front Mol Neurosci. 2016 Dec 26;9:145. doi: 10.3389/fnmol.2016.00145. eCollection 2016. Front Mol Neurosci. 2016. PMID: 28082864 Free PMC article.
-
Derivation of Specific Neural Populations From Pluripotent Cells for Understanding and Treatment of Spinal Cord Injury.Dev Dyn. 2019 Jan;248(1):78-87. doi: 10.1002/dvdy.24680. Epub 2018 Nov 26. Dev Dyn. 2019. PMID: 30324766 Free PMC article. Review.
-
Spinal lumbar dI2 interneurons contribute to stability of bipedal stepping.Elife. 2021 Aug 16;10:e62001. doi: 10.7554/eLife.62001. Elife. 2021. PMID: 34396953 Free PMC article.
-
The late and dual origin of cerebrospinal fluid-contacting neurons in the mouse spinal cord.Development. 2016 Mar 1;143(5):880-91. doi: 10.1242/dev.129254. Epub 2016 Feb 2. Development. 2016. PMID: 26839365 Free PMC article.
References
-
- Berkowitz A (2001) Rhythmicity of spinal neurons activated during each form of fictive scratching in spinal turtles. Journal of neurophysiology 86: 1026–1036. - PubMed
-
- Cazalets JR (2000) Organization of the spinal locomotor network in neonatal rat. In: Inc Hp, editor. Neurobiology of spinal cord injury: R. Kalb and S.M. Stritmatter. pp. 89–111.
-
- Armstrong DM (1986) Supraspinal contributions to the initiation and control of locomotion in the cat. Progress in neurobiology 26: 273–361. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

